What is Epinephrine Market Size?
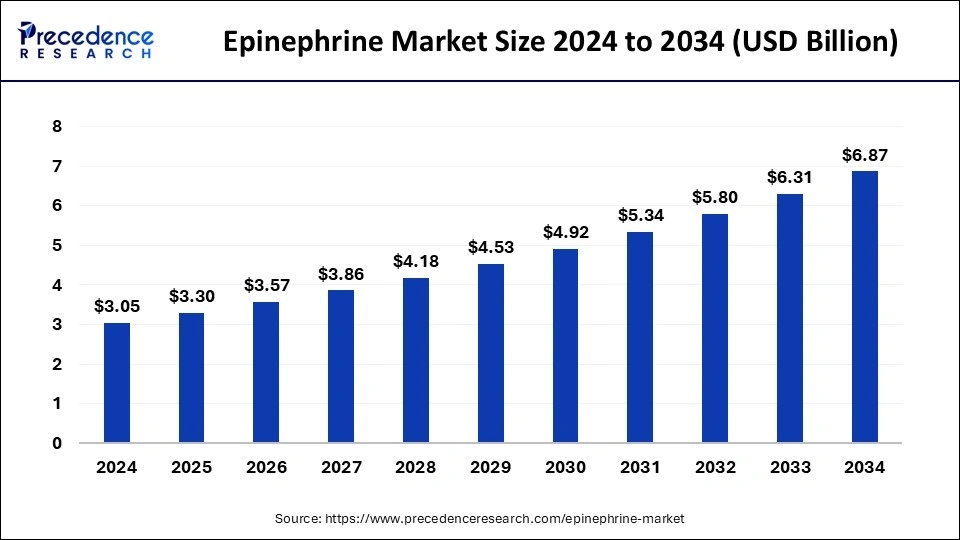
The global epinephrine market size accounted for USD 3.30 billion in 2025 and is predicted to increase from USD 3.30 billion in 2025 to approximately USD 6.87 billion by 2034, expanding at a CAGR of 8.50% from 2025 to 2034. One of the major factors driving the revenue growth trajectory of the global epinephrine market is the growing incidence of anaphylaxis, primarily in the age group of kids across the globe. For instance, as per the analysis by Pubmed Central, nearly 5% of the U.S. populace has suffered anaphylaxis. Further, prominent players in the epinephrine market are focused on launching cost-effective generic epinephrine products, which in turn are expected to create lucrative revenue growth opportunities in this market.
Market Highlights
- North America acquired a major revenue share of 33.45% in 2024.
- Asia-Pacific will likely account for a sizable portion of the market from 2025 to 2034.
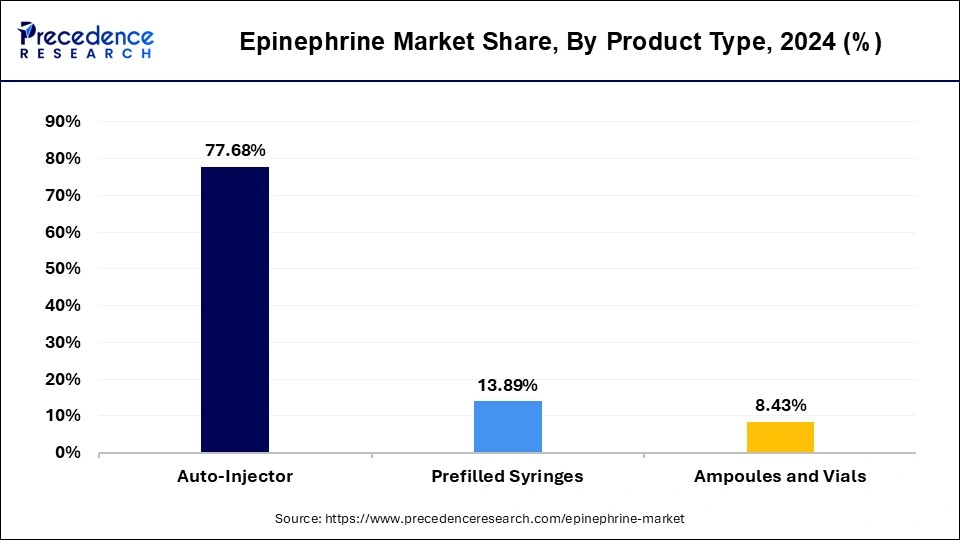
- By product type, the Autoinjectors segment held the major share of around 77.68% in 2024.
- By application, the cardiac arrest segment is predicted to grow quickly from 2025 to 2034.
- By distribution channel, the retail pharmacies segment held the highest share of 40.83% in 2024.
- By distribution channel, the online pharmacies segment is predicted to grow fastest from 2025 to 2034.
Market Size and Forecast
- Market Size in 2025: USD 3.30 Billion
- Market Size in 2026: USD 3.57 Billion
- Forecasted Market Size by 2034: USD 6.87 Billion
- CAGR (2025-2034): 8.50%
- Largest Market in 2024: North America
- Fastest Growing Market: Asia Pacific
MarketOverview
For many years, thousands of people of all ages have benefited from Epinephrine. Because Epinephrine acts as a hormone and medicine, its use has rapidly risen in various contexts. The global epinephrine market is expanding due to the FDA's approval of its usage in several scenarios, including the urgent treatment of type 1 hypersensitivity reactions, maintaining mydriasis during intraocular procedures, and hypertension brought on by septic shock.
The prevalence of outpatient prescriptions, the frequency of anaphylaxis cases, the rise in food allergy cases, the use of Epinephrine during hand surgery, the use of Epinephrine by plastic surgery-trained surgeons, the majority of regulatory agencies and medical professionals authorizing Epinephrine because of its safe usage, the background of training, the location, and the practice setup are the main variables impacting the worldwide epinephrine market.
The use of Epinephrine is increasing quickly in a variety of medical settings due to its advantages, which include the ability to give advanced cardiovascular life support to patients, improved neurologic outcome, rapid absorption, heart stimulation, improved breathing, balanced blood pressure, reduced face swelling, including lips and throat, and first line cure for allergic reactions that are life-threatening. Additionally, the body's response to allergens is slowed down by adrenaline.
Epinephrine is allowed for use, although there are potential side effects linked with it. Additionally, recent research has demonstrated that Epinephrine may negatively affect the central nervous system, gastrointestinal tract, neuromuscular system, respiratory system, renal system, cardiovascular system, and neuromuscular system. According to researchers, Epinephrine is a significant cause of adverse effects on critical bodily components and results in medication interactions. Regulatory agencies have generally disregarded Epinephrine as a crucial agent in operating room (OR) settings, particularly for the local anesthetic block. Despite its intended utility, studies have shown that Epinephrine can adversely affect humans and the environment, significantly impacting the market situation.
Other barriers to the growth of the global epinephrine market include the high cost of auto-injectors and the limited availability of reimbursement in underdeveloped countries.
Epinephrine MarketTrends
- The growing demand for personalized customer experiences
- Technological advancements
- The integration of artificial intelligence (AI)
- Strategic investment in automation
- Infrastructure development
- Rising consumer spending
What is the Role of AI in the Epinephrine Market?
Artificial intelligence (AI) and machine learning (ML) can analyze high datasets to identify patterns and predict anaphylactic episodes, improve diagnostic accuracy. Epinephrine is a highly effective treatment for anaphylaxis, and the only recommended first-line medication in anaphylaxis management. AI benefits also include reduces errors and mistakes, easily handles big data, data privacy concerns, customer support, automating processes, availability, better decisions, bolstered cybersecurity, scalability, predictive maintenance, medical advances, completing repetitive tasks, boosts productivity, fraud detection, automate repetitive tasks, innovation, AI complements humans, and advanced data analysis.
Market Outlook
- Industry Outlook: The industry is shifting toward two parallel pathways: commoditized hospital-supply generics and differentiated consumer-facing auto-injector solutions. Manufacturers are optimizing cold- and room-temperature supply chains, improving device ergonomics, and investing in patient education and distribution channels. Regulatory scrutiny remains high driven by the critical safety profile of emergency drugs so quality systems and pharmacovigilance are paramount. Partnerships between pharma companies, device engineers, and emergency-response NGOs are likely to intensify, focusing on training, public access defibrillator/epinephrine placement strategies, and secure supply in low-resource settings. Consolidation and strategic licensing deals will continue as companies seek scale in manufacturing and device integration.
- Sustainability Trend: Sustainability considerations are emerging in packaging, manufacturing footprint, and device lifecycle management. Key trends include: recyclable or reduced-plastic packaging for single-use devices, procurement choices favouring manufacturers with lower-energy manufacturing footprints, and designing auto-injectors for easier, safer disposal or take-back programs to reduce medical waste. Some manufacturers are exploring materials with lower embodied carbon and supply-chain consolidation to minimise transport emissions. While epinephrine's life-saving function dominates procurement decisions, sustainability credentials are progressively becoming a differentiator for large healthcare purchasers and institutional tenders.
- Major Investment: Major investment areas focus on, user-friendly, fail-safe auto-injector designs with improved ergonomics, visual/audible feedback and simplified instructions for untrained bystanders. extended-stability formulations to lengthen shelf life and reduce replacement frequency. sterile-fill capacity expansions, automated aseptic lines, and contingency stocks for emergency supply reliability. expanding public-access programs, school/municipal stockpiles, and supply initiatives in emerging economic. investments into training platforms, apps for emergency guidance, and telehealth integration for allergy management. Philanthropic and public private funds may also support access programs in low-resource regions and surge-capacity manufacturing.
- Startups: Startups in the epinephrine ecosystem are mostly device- and delivery-focused. Typical innovation vectors include novel delivery platforms, needle-free or minimally invasive intranasal/sublingual systems aimed at layperson administration. Disposable, low-cost auto-injectors engineered for mass public placement schools, stadiums. Affordable manufacturing-as-a-service platforms helping smaller players produce aseptically filled injectables or devices at lower capital outlay.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 3.30 Billion |
| Market Size in 2026 | USD 3.57 Billion |
| Market Size by 2034 | USD 6.87 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 8.50% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Segments Covered | By Type, By Application, and By Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Technological advancement
Technological advancements in epinephrine include an epinephrine autoinjector which is a medical device for injecting a measured dose or doses of epinephrine by means of autoinjector technology. Epinephrine treats severe allergic reactions (anaphylaxis) or sudden asthma attacks. It is also used to treat slow heart rate and low blood pressure. It reduces the effects of an allergic reaction like trouble in breathing or swelling of the throat, lips, and face.
Restraint
Data privacy regulations
Privacy policy is living documents that must accurately reflect your current data collection and processing activities. Otherwise, it directly violet data privacy laws and risk falling behind as more laws take effect. Data privacy risks include social engineering attacks, phishing scams, online tracking, data breaches, and identity theft. Common adverse effects of epinephrine include tremors, weakness, vomiting, nausea, diaphoresis, palpitations, apprehension, anxiety, headache, hypertension, and tachycardia.
Opportunity
Investing in secure data infrastructure:
The investing in secure data infrastructure include greater visibility, disaster recovery, data encryption, cloud compliance, security, cybersecurity risks, collaboration, compliance, scalability, data protection, and cost savings. Digital infrastructure is the key to streamlining business processes, enhancing customer service, reducing operational costs, and creating a competitive benefit. The benefits of digital infrastructure include cost savings, customer experience, communication & collaboration, and automation.
Segment Insights
Product Type Insights
The autoinjector segment held a dominant presence in the epinephrine market in 2024.
- In May 2025, Pyzchiva autoinjector, first commercially available in Europe for Ustekinumab biosimilars was launched by Sandoz.
The prefilled syringes segment is expanded to grow at the fastest rate in the market during the forecast period of 2025 to 2034.
- In January 2025, next generation polymer syringe system, SCHOTT TOPPAC was launched by SCHOTT Pharma, a provider of drug containment and delivery solutions.
The prefilled syringes segment is expected to grow the fastest in the coming years. Prefilled syringes offer a convenient way to administer epinephrine to patients experiencing cardiac arrest. In some countries, adrenaline is available only in glass ampoules. However, simplification of cardiopulmonary resuscitation (CPR) by introducing prefilled syringes may ensure more efficient CPR. The aim of this study was to investigate the impact of different forms of adrenaline on CPR quality.
Application Insights
The anaphylaxis segment accounted for a considerable share of the epinephrine market in 2024.
- In November 2024, to deliver allergy and anaphylaxis training for school staff was launched by Allergy UK in collaboration with the Allergy Team. It covers how to prevent allergic reactions as well as how to respond to them.
The cardiac arrest segment is projected to experience the highest growth rate in the market between 2025 and 2034.
- In August 2025, health advisory to prevent sudden cardiac arrest among gym goers and sportspersons was launched by Punjab Government.
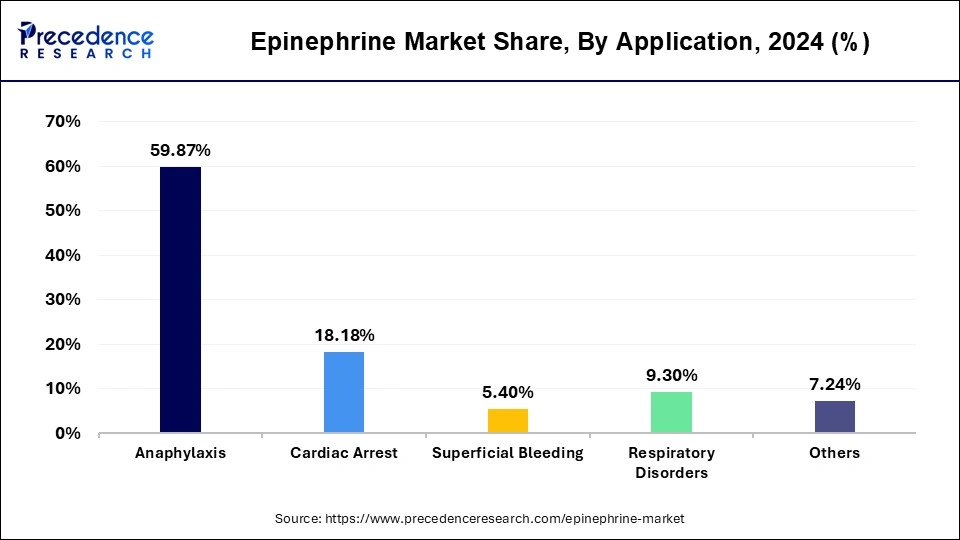
Epinephrine Market Revenue, By Application, 2022-2024 (USD Billion)
| By Application | 2022 | 2023 | 2024 |
| Anaphylaxis | 1,571.5 | 1,693.7 | 1,826.8 |
| Cardiac Arrest | 469.7 | 510.3 | 554.8 |
| Superficial Bleeding | 143.3 | 153.7 | 164.9 |
| Respiratory Disorders | 242.1 | 262.0 | 283.7 |
| Others | 193.0 | 206.5 | 221.0 |
Distribution Channel Insights
The retail pharmacies segment led the market.
- In April 2025, new e-commerce experience for retail pharmacies was introduced by GoodRx, the leading platform for medication savings in the U.S.
The online pharmacies segment is set to experience the fastest rate of the market growth from 2025 to 2034.
- In August 2025, online pharmacy, promises 10-minute medicine delivery check areas covered was launched by quick commerce platform, Zepto.
Epinephrine Market, By Distribution Channel, 2022-2024 (USD Billion)
| By Distribution Channel | 2022 | 2023 | 2024 |
| Hospital Pharmacies | 815.9 | 877.6 | 944.7 |
| Retail Pharmacies | 1,082.6 | 1,160.9 | 1,245.9 |
| Online Pharmacies | 721.0 | 787.5 | 860.6 |
Regional Insights
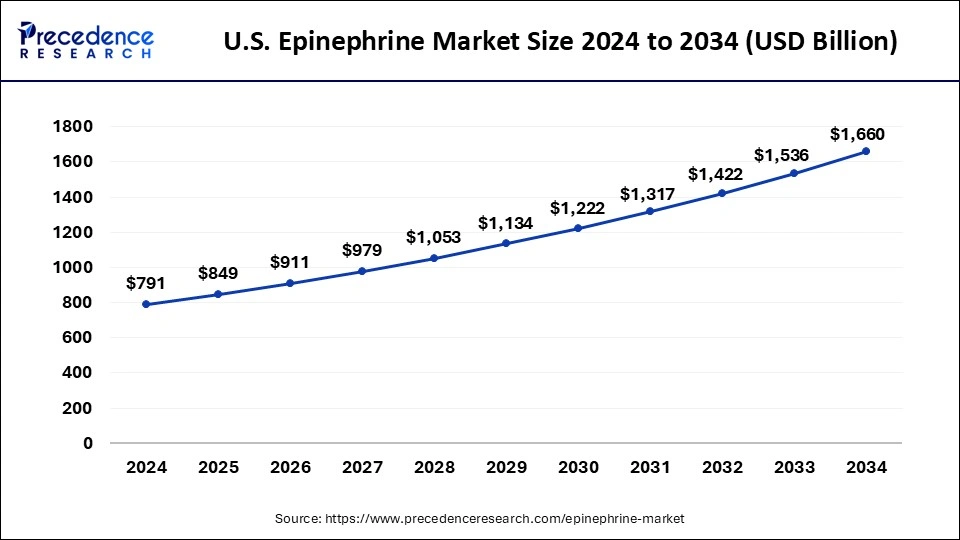
U.S.Epinephrine Market Size and Growth 2025 to 2034
The U.S. epinephrine market size is evaluated at USD 849 million in 2025 and is projected to be worth around USD 1,660.0 million by 2034, growing at a CAGR of 7.70% from 2025 to 2034.
The epinephrine market in North America will likely develop significantly over the following years due to the region's expanding patient population and rising medical science technological advancements. Numerous ongoing research and development initiatives in the U.S. will positively affect the epinephrine industry in the area.
North America acquired the largest revenue share in the year 2024owing to better research & development programs & initiatives, high-quality healthcare infrastructure, and better reimbursement policies in the region. U.S. is the leading country, followed by Canada, and Mexico. The U.S. has recorded the highest sale in the market in the year. While the region's primary healthcare emphasis remains on the pandemic as it moves through the 3rd year of its disruptive impacts and the death toll which approached to 1 million, other key dynamics are playing out with respect to health services utilization, the associated level of spending including patient costs out-of-pocket, and the use of prescription medicines. Understanding these factors of the healthcare system and how they may develop over the next few years remains critical to stakeholders and decision-makers including patients.
Furthermore, in order to fulfil the need of epinephrine in Canada, Bausch Health, Canada, which is part of Bausch Health Companies Inc. announced in, that Emerade™ sterile epinephrine solution for injection in a pre-filled pen) will be available across Canada for the emergency treatment of anaphylactic reactions in patients who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions. Emerade offers the most affordable EAI in Canada for anaphylaxis with the lowest price in the majority of territories and provinces.
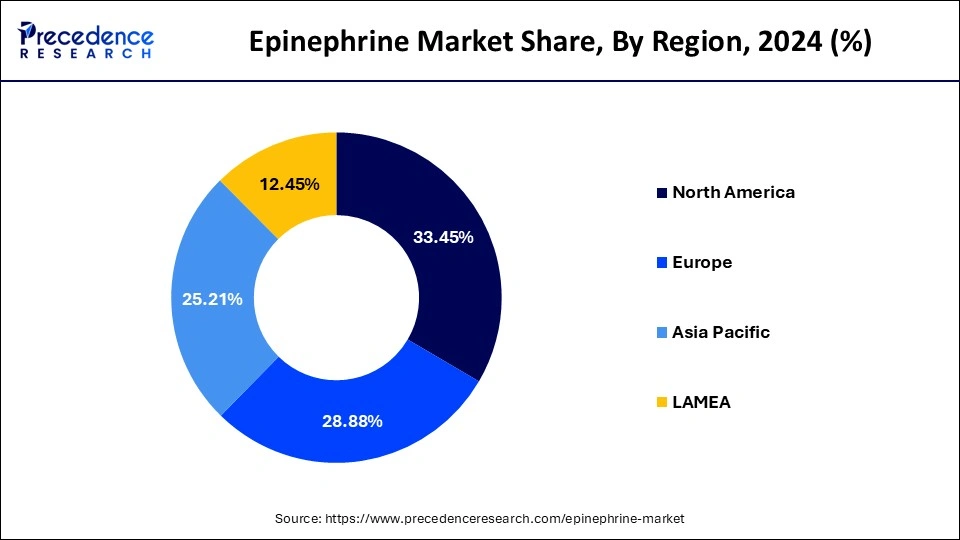
During the forecast period, Asia-Pacific will likely account for a sizable portion of the market.The main drivers of the expansion of the epinephrine business are escalating technology advancement and medical advancement in China, Taiwan, and South Korea. Asia Pacific is expected to grow fastest in the forecast period, owing to better research & development programs & initiatives, high-quality health care infrastructure, and better reimbursement policies in the region. China is the leading country, followed by India, and Japan. Penetration of generic drugs is largest in China, with India being the fastest growing. Health systems across the region are leveraging generic medications increasingly to minimize costs. The rising trend with respect to manufacturing of epinephrine in Asia Pacific is not just the consequence of cost-containment moves by the government, but also since the national governments identify the advantages of having an indigenous manufacturing base locally rather than importing.
Asia Pacific's significance to pharmaceutical enterprises' global marketing and product strategies should not be neglected. The aging population locally and expanded government commitment to serve affordable healthcare are determinants facilitating the demand for epinephrine in the region. According to APAAACI 2019, the overall prevalence of food allergy in pre-schoolers was only 1% in Thailand, but as high as 5.3% in Korean infants and 3.8% and 7.7% in one-to two-year-old children in China respectively. Wheat allergy is particularly prominent in Japan, Korea and Thailand. In Japanese school children, wheat allergy is more common than nuts and shellfish and is the main cause of food anaphylaxis in both Japan and Korea. These factors are ought to grow the epinephrine market in the region.
North America dominated the global epinephrine market in 2024.
In September 2024, the U.S. availability of neffy (epinephrine nasal spray), the first and only needle-free treatment for Type-1 allergic reactions including Anaphylaxis was announced by ARS Pharmaceuticals, Inc., a biopharmaceutical company dedicated to empowering at risk patients and their caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis.
Asia Pacific is anticipated to grow at the fastest rate in the market during the forecast period.
- In August 2023, publication of positive clinical results with FMXIN002 intranasal powder epinephrine spray in the journal of allergy and clinical immunology in practice was announced by Nasus Pharma Ltd, a clinical stage biopharmaceutical company focused on developing needle-free, powder-based intranasal (PBI), product portfolio, to address acute medical conditions.
| Region/Country | Regulatory Body | Key regulations | Market focus areas |
| Germany | Fedral institute for drugs and medical devices | Strict, GMP, device usability requirements | Hospital emergency supply, pharmacy distribution in auto injector safety |
| UK | MHRA | Device and drug combo approvals and emergency use | School and public access programs |
| France | ANSM | High device testing standrads | Community use, hospital emergency kits, allergy |
| Italy | AIFA | Regional procurement controls | Hospitals and EMS supply, community allergy programs |
Recent Developments
- In February 2025, the generic version of Epinephrine injection multiple dose vial in the US, which is eligible for 180 days of competitive generic therapy (CGT) exclusivity was launched by Glenmark Pharmaceuticals. Epinephrine injection of strength 10 mg/mL (1 mg/mL) multiple-dose vial.
- In December 2024, generic epinephrine injection in U.S. was launched by Fresenius Kabi. Epinephrine injection, USP is a prescription medicine used to increase mean arterial blood pressure in adult patients with hypotension associated with septic shock for emergency treatment of allergic reactions, including anaphylaxis and for induction and maintenance of mydriasis during intraocular surgery.
Market Value Chain Analysis
- Raw Material Sourcing: Epinephrine production relies on pharmaceutical-grade active pharmaceutical ingredient (API) feedstocks and high-purity excipients. Sourcing emphasizes reliability, batch-to-batch consistency, and supplier qualification under stringent GMP frameworks.
- Technological Advancements:Technological progress in the epinephrine market spans both pharmacology and device engineering. On the formulation side, advances aim to increase stability reducing oxidative degradation and allow room-temperature storage with longer shelf-lives.
Key Players in Epinephrine Market and Their Offerings

- Abbott Laboratories: An American multinational medical devices and health care company headquartered in Abbott Park, Illinois.
- Sanofi S.A.: A French multinational pharmaceutical company that develops and manufactures medicines and vaccines.
- Mylan, Inc.: A pharmaceutical company that has since merged with Pfizer's Upjohn to form Viatris. It was founded in 1961 and was a major producer of generic drugs and over-the-counter products.
- ALK Abello A/S: A Denmark-based company focused on allergy research and treatment.
- Amedra Pharmaceuticals LLC: A U.S.-based specialty pharmaceutical company.
- Lincoln Medical, Ltd.: Likely a reference to Lincoln Pharmaceuticals, an Indian company that develops and markets affordable medicines. It received a patent for an anti-malarial drug and has facilities certified by the European Union.
Segments Covered in the Report
By Product Type
- Prefilled Syringe
- Auto-injector
- Ampoules and Vials
By Application
- Anaphylaxis
- Cardiac Arrest
- Superficial Bleeding
- Respiratory Disorders
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Tags
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting