Controlled Release Drug Delivery Market Will Grow at CAGR of 9.26% By 2032
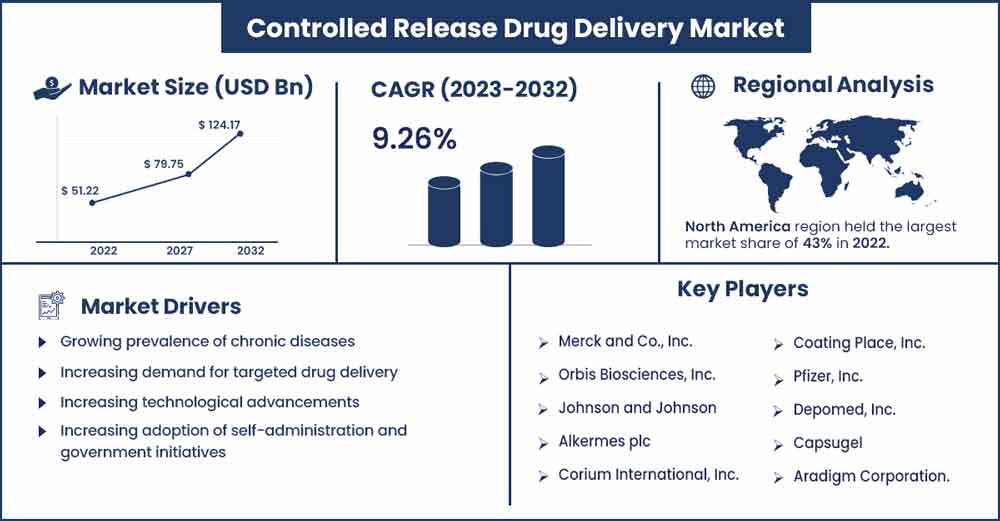
The global controlled release drug delivery market size is anticipated to reach around USD 124.17 billion by 2032 up from USD 51.22 billion in 2022 with a CAGR of 9.26% Between 2023 and 2032.
A controlled drug administration system ensures patient compliance, increases treatment effectiveness and ensures patient safety. Any delivery method that produces a delayed medicine release over a long period is considered a controlled release system. Less frequent dosage and improved plasma drug level management are used to achieve this. A controlled release formulation's successful commercialization is frequently tricky and requires considering various elements, including the drug's physiochemical qualities, physiological considerations, and manufacturing variables. Developing a medicine's controlled release drug delivery system aims to maximize therapeutic advantages while minimizing adverse effects and managing the sick condition.
When the market for controlled-release pharmaceuticals entered the pharmaceutical industry, the hunt for an original solution to the issues related to drug delivery came to an end. The pharmaceutical industry has recently shown a lot of admiration for the new and mechanistic approach used by controlled-release medications in the treatment regimen. Maintaining the patient's medicine concentration within the targeted range is more important to the market. The market guarantees significant benefits like biocompatibility and non-toxicity of the elements used to administer drugs to the patient.
The development of innovative delivery platforms that can be tuned propels the production of a powerful, strong, and fantastic pipeline of controlled-release medications. These medications treat broad-spectrum illnesses like infectious diseases, cancer, inflammatory disorders, autoimmune diseases, and neurological affections.
Existing controlled-release medications, such as those classified as glucagon-like peptide-1 (GLP-1) analogs for diabetes or tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis (RA), have entirely transformed the pharmaceutical industry by raising the survival rate of patients who had previously experienced severe toxicity and side effects as a result of the drug's unintended concentration inside the body.
The controlled release mechanism has become one of the most extensively used and admired drug delivery systems due to the efforts put forth in the growth of the market and the criteria linked to it. To increase sales of new and existing medications for important indications, pharma developers are now switching to controlled release mechanisms of drug delivery. Additionally, reduced side effects, less toxicity, and maximum precision are a few of the reasons why controlled-release medications continue to gain favor with both researchers and patients. According to estimates, introducing control release technology as a crucial enabling technology in the therapeutics industry is driving the market toward success, which researchers and patients have long needed.
The introduction of the drug delivery market has brought about an elementary change in the pharmaceutical industry. It has eliminated all barriers in the prior markets, causing patients to develop unintended side effects. The market is drawing in several new patients due to the extensive applications that older patients have gotten, which will likely raise the market's standards regarding advantages and benefits. According to the market analysis, researchers and clinicians will likely produce safe and effective controlled-release medications that would supply the market with customized pharmacotherapeutic regimens, resulting in widespread market acceptance. Additionally, the market's strong clinical pipeline and its effect on delivering the desired concentration of medicine point to potential high-end growth across all indications.
Report Highlights:
- By technology, Targeted delivery held the most significant market share of more than 24% in 2022. Targeted delivery allows medications to be placed away from areas where they can cause drug toxicity. Implantable devices guarantee linear medication delivery while improving efficiency, reducing adverse effects, and providing convenience.
- By release mechanism, chemically activated delivery systems have become extremely popular in recent years. The ability to quickly and precisely react to the target metabolite, leading to enhanced pharmacological therapy, is causing the segment to flourish. For instance, an enzyme-activated release mechanism achieves optimal and controlled insulin release in hyperglycemic patients.
- By application, the oral-controlled segment had a share of more than 37% of total revenue in 2022. Another attractive market that will likely expand significantly is meter dose inhalers. Long-acting injectables are preferred to conventional delivery forms because they have several benefits, such as a predictable drug release profile.
- By region, North America was the most important regional market globally, with a market share of more than 43% in 2022. The burden of chronic illnesses like cancer, diabetes, and heart ailments is rising, and Asia Pacific is likely to have the greatest CAGR in the pharmaceutical industry.
Controlled Release Drug Delivery Market Report Scope:
| Report Coverage | Details |
| Market Revenue in 2023 | USD 55.96 Billion |
| Projected Forecast Revenue in 2032 | USD 124.17 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 9.26% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Regional Snapshot:
North America was the most important regional market globally, with a market share of more than 42.8% in 2021. Government programs that encourage the development of controlled-release drug delivery and rising R&D spending will likely fuel market expansion in the area. In addition, the presence of significant market participants helps to encourage the use of controlled-release medication delivery systems. The burden of chronic illnesses like cancer, diabetes, and heart ailments is rising, fostering market expansion. One of the major factors driving the expansion of the regional market is the spiralling use of controlled-release medication delivery systems for hypertension treatment.
Due to increased research and development activities and significant regional pharmaceutical businesses, Europe accounted for the second-largest share in 2022. With the considerable increase in patients with chronic illnesses like diabetes, cancer, and COPD, the local market will likely experience substantial expansion during the ensuing years. The predicted period is likely to have the greatest CAGR in Asia Pacific. The region has been attracting attention worldwide as the pharmaceutical industry continues to grow. The market in the region is benefiting from the pharmaceutical industries' rapid expansion in China and India. Another significant element driving product acceptance in Japan is the country's sizable elderly population.
Market Dynamics:
Market Drivers:
Several drivers will likely shape the controlled-release drug delivery market in the coming years. Some of these drivers include:
- Growing prevalence of chronic diseases: The increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular diseases is one of the major drivers for the controlled-release drug delivery market. These diseases require long-term treatment facilitated through controlled-release drug delivery systems.
- Increasing demand for targeted drug delivery: The use of targeted drug delivery systems to treat cancer and other chronic diseases has increased. This is because they allow drugs to be delivered directly to the disease site, which can reduce the side effects of the treatment and increase its effectiveness.
- Technological advancements: The controlled-release drug delivery market will likely benefit from developing new technologies such as nanotechnology and smart polymers, which are used to develop more advanced and efficient controlled-release drug delivery systems.
- Government initiatives: Governments worldwide are increasingly investing in research and development in the field of drug delivery, which will likely drive the growth of the controlled-release drug delivery market.
- Increasing adoption of self-administration: The increasing trend towards self-administration of drugs will likely drive the demand for controlled-release drug delivery systems, as these systems allow patients to administer the drugs themselves without the need for frequent visits to a healthcare provider.
- Growing demand for generic drugs: The growing demand for generic drugs will drive the controlled-release drug delivery market. These drugs are formulated using controlled-release drug delivery systems to extend their shelf life and improve their effectiveness.
Market Restraints:
- High cost of drug delivery systems: The development and manufacturing of controlled-release drug delivery systems can be expensive, limiting their adoption by healthcare providers and patients.
- Complex regulatory environment: The regulatory environment for drug delivery systems can be complicated, creating barriers to entry for new players in the market.
- Limited efficacy of some drug delivery systems: Some controlled-release systems may not be effective in delivering certain drugs, which can limit their adoption by healthcare providers.
- Competition from alternative drug delivery systems: The controlled release drug delivery market will likely face competition from alternative drug delivery systems such as traditional oral tablets and injections.
- Limited patient compliance: Patient compliance with drug regimens can be challenging, limiting the effectiveness of controlled-release drug delivery systems.
- Lack of awareness: There may be a need for more understanding about the benefits of controlled-release drug delivery systems among healthcare providers and patients, limiting their adoption.
Major Key Players:
- Merck and Co., Inc.
- Orbis Biosciences, Inc.
- Johnson and Johnson
- Alkermes plc
- Corium International, Inc.
- Coating Place, Inc.
- Pfizer, Inc.
- Depomed, Inc.
- Capsugel
- Aradigm Corporation
Market Segmentation:
By Technology
- Coacervation
- Wurster Technique
- Implants
- Micro Encapsulation
- Targeted Delivery
- Transdermal
- Others (Liposomes, Microelectromechanical Technology)
By Release Mechanism
- Partition Controlled Micro Reservoir Drug Delivery Systems
- Polymer Based Systems
- Drug Delivery Systems, which are Feedback Regulated
- Drug Delivery Systems that are Activation-modulated
- Hydrodynamic Pressure Activated
- Osmotic Pressure Activated
- Magnetically Activated
- Vapor Pressure Activated
- Mechanically Activated
- Chemically Activated
- Hydrolysis Activated
- pH Activated
- Enzyme Activated
By Application
- Injectable
- Metered Dose Inhalers
- Infusion Pumps
- Ocular and Transdermal Patches
- Oral Controlled-drug Delivery Systems
- Eluting Drug Stents
Buy this Research Report@ https://www.precedenceresearch.com/checkout/2567
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 9197 992 333