Narcolepsy Therapeutics Market Will Grow at CAGR of 7.70% By 2032
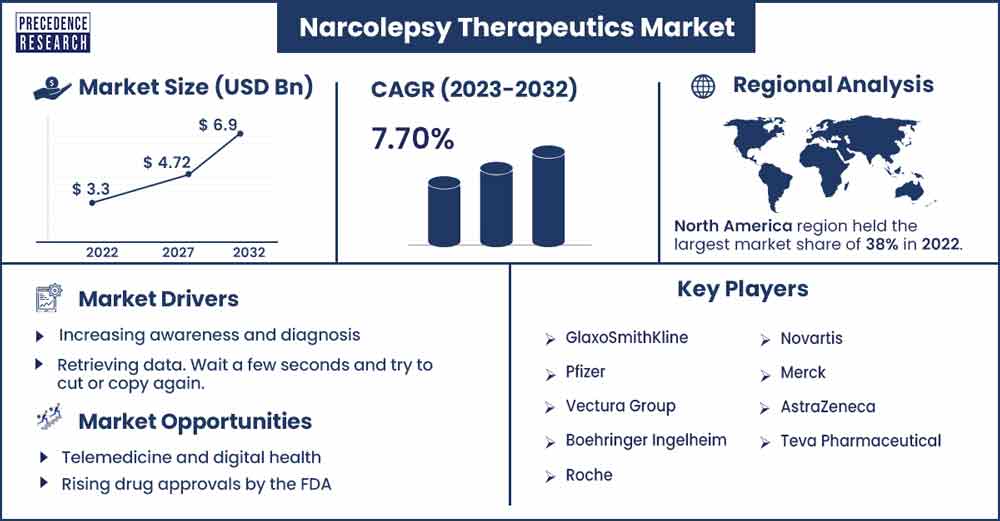
The global narcolepsy therapeutics market size was exhibited at USD 3.3 billion in 2022 and is anticipated to touch around USD 6.9 billion by 2032, expanding at a CAGR of 7.70% from 2023 to 2032.
Market Overview
Narcolepsy is a rare chronic neurological disorder that affects an individual brain's ability to control sleep-wake cycles. In this health condition, the brain cannot control the ability to sleep or stay awake for longer periods. People with narcolepsy suffer from uncontrollable daytime sleepiness and may suddenly fall asleep during any type of activity. Narcolepsy affects both females and males.
The most common symptoms of Narcolepsy disorder include excessive daytime sleepiness (EDS), cataplexy, hallucinations, automatic behaviors, and sleep paralysis. Narcolepsy is often diagnosed through a sleep study or different types of medical tests requiring an overnight stay in a sleep lab. To treat narcolepsy healthcare providers may prescribe a type of medicine known as a stimulant, such as pitolisant, modafinil, solriamfetol, methylphenidate, dextroamphetamine sulfate, and others.
Growth Factors
The growth of the global narcolepsy therapeutics market is attributed to the growth in healthcare infrastructure, increasing demand for personalized medicine, rise in drug discovery and development, increase in clinical trials, supportive government initiatives, and rising investment in research and development activities. In addition, the market is growing as a result of the increasing cases of narcoleptic disorders, rising stress levels, increasing aging population, and growing awareness about numerous effective treatment options.
- In February 2023, Axsome Therapeutics, Inc. and Pharmanovia signed a strategic licence agreement for the commercialization and development of Sunosi (solriamfetol), the first and only dual-acting dopamine and norepinephrine reuptake inhibitor that has been demonstrated to increase wakefulness in adults with narcolepsy or obstructive sleep apnea (OSA)-related excessive daytime sleepiness (EDS) in Europe and some Middle Eastern and North African (MENA) nations. Axsome is eligible to receive sales-based and other milestone payments totaling up to USD 101 million, in addition to the upfront payment of USD 66 million.
- NLS Pharmaceutics Ltd. declared in May 2023 that the FDA in the United States had given the go-ahead to start the Quilience (Mazindol ER) Phase 3 Clinical programme. Two double-blind Phase 3 trials (N=50 each) comparing Mazindol ER to placebo in adult narcoleptic patients will be part of the AMAZE Programme. These trials will start this summer at various locations across the US.
- In December 2023, Bristol Myers Squibb announced the acquisition of Karuna Therapeutics intending to strengthen its neuroscience portfolio. Bristol Myers Squibb has agreed to acquire Karuna for a total equity value of USD 14.0 billion or USD 12.7 billion net of estimated cash acquired.
Regional Insights
North America is expected to sustain its dominance in the upcoming years. The growth of the region is driven by the presence of a well-established healthcare infrastructure, high research and development expenditure, supportive reimbursement policies for therapeutic products, and the rising number of pharmaceutical companies.
The presence of major companies operating in the region is expected to fuel the market’s expansion including Harmony Biosciences (BIOPROJET), XWPharma Ltd., Axsome Therapeutics Inc., Ligand Pharmaceuticals Incorporated, Teva Pharmaceuticals USA, Inc. and others. The United States market experiencing robust growth due to the increasing healthcare expenditure, the presence of a strong clinical pipeline, increased adoption of narcolepsy therapeutics, and rising awareness regarding advanced therapies. In addition, advancements in technology occurring in medical diagnosis are likely to fuel the market’s expansion.
Furthermore, the rising prevalence of narcolepsy creates the need for narcolepsy therapeutics for the treatment which results in accelerating the growth of the market. For instance, according to the report published by the Narcolepsy Network, Inc. in 2023, Narcolepsy disorder affects nearly 1 in every 2,000 people in the U.S. Symptoms of narcolepsy include excessive daytime sleepiness (EDS), sleep attacks, cataplexy, hallucinations, sleep paralysis, disrupted nighttime sleep, and others. Several researchers have found a gene that is linked to narcolepsy.
Nearly one-quarter of the population in the United States carries the genetic marker for narcolepsy, but only 1 person in 500 of these people will develop narcolepsy. Narcolepsy can be treated either with medications or changes in lifestyle which has proved to be effective in treating the disorder. Thus, this is expected to propel the market growth in the region during the forecast period.
Narcolepsy Therapeutics Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 3.54 Billion |
| Projected Forecast Revenue by 2032 | USD 6.9 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 7.7% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Rising incidence of narcolepsy disorders
The rising prevalence of narcolepsy across the globe has led to the rising adoption of narcolepsy treatments. Narcolepsy is a chronic neurological sleep disorder that includes excessive attacks of drowsiness during the daytime, also known as excessive daytime sleepiness (EDS). In many cases, it causes unexpected and temporary loss of muscle control, which is commonly referred to as cataplexy.
For instance, according to the Narcolepsy Network, Inc. Narcolepsy affects 200,000 Americans and nearly 3 million globally. It is projected that only 25 percent of patients who have narcolepsy have been diagnosed and are receiving appropriate treatment. There are many classes of medications healthcare providers to treat narcolepsy, such as Alerting agents, Serotonin-norepinephrine reuptake inhibitors (SNRIs), Selective serotonin reuptake inhibitors (SSRIs), Tricyclic antidepressants, Oxybates (Xyrem and Xywave), Pitolisant (Wakix), Solriamfetol (Sunosi), and others. Therefore, with increasing cases of narcolepsy, the demand for narcolepsy therapeutics is anticipated to fuel the growth of the global narcolepsy therapeutics market in the coming years.
Restraint
Lack of infrastructure
The lack of proper healthcare infrastructure in developing and under-developing countries is projected to restrain the market’s expansion during the forecast period. Less technology penetration in such countries may adversely impact the medical diagnosis infrastructure and is likely to limit the expansion of the narcolepsy therapeutics market during the forecast period.
Opportunities
Rise in R&D activities
The ongoing research and development activities coupled with increasing healthcare infrastructure for narcolepsy therapeutics are expected to offer a lucrative opportunity for market growth during the forecast period. The market is witnessing sophisticated healthcare infrastructure due to an increase in healthcare investment. Therefore, advanced healthcare facilities offer effective treatment for narcolepsy disease and are expected to contribute to the market’s growth.
Rising drug approvals by the FDA
The increase in drug approvals by the U.S. Food and Drug Administration is expected to offer lucrative opportunities for the market. The researchers are consistently working towards discovering advanced drugs to treat patients with Narcolepsy diseases to provide a healthy life. For instance, In May 2023, the U.S. Food and Drug Administration approved Lumryz (sodium oxybate) for the treatment of cataplexy or excessive daytime sleepiness in adults suffering with narcolepsy. Moreover, the robust growth of the pharmaceutical sector has resulted in an increasing number of drug discoveries and effective commercialization of therapeutics. Thereby bolstering the market’s growth.
Recent Developments
- In October 2023, Jazz Pharmaceuticals plc announced that the Company and its partners present 14 abstracts from across its sleep medicine portfolio, including two oral presentations, at the 17th annual World Sleep 2023 Congress, held October 20-25 in Rio de Janeiro, Brazil.
- In October 2023, West Virginia University (WVU) joined the BRAIN Center to advance technology for neurological disorders. WVU will serve as the core clinical testing site within a consortium of top universities and industries to develop technologies designed to improve the care and rehabilitation of people with neurological disorders.
Key Market Players
- Avadel Pharmaceuticals
- Harmony Biosciences (BIOPROJET)
- Jazz Pharmaceuticals PLC
- Ligand Pharmaceuticals Incorporated
- Novartis AG
- Shionogi Inc.
- Takeda Pharmaceutical Company
- Teva Pharmaceuticals Industries Ltd
- Axsome Therapeutics Inc.
- XWPharma Ltd.
Market Segmentation
By Treatment
- Narcolepsy With Cataplexy
- Narcolepsy Without Cataplexy
- Secondary Narcolepsy
By Product
- Central Nervous System Stimulants
- Sodium Oxybate
- Selective Serotonin Reuptake Inhibitor
- Tricyclic Antidepressants
- Others
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3410
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308