Bowel Stimulators Market Size and Forecast 2025 to 2034
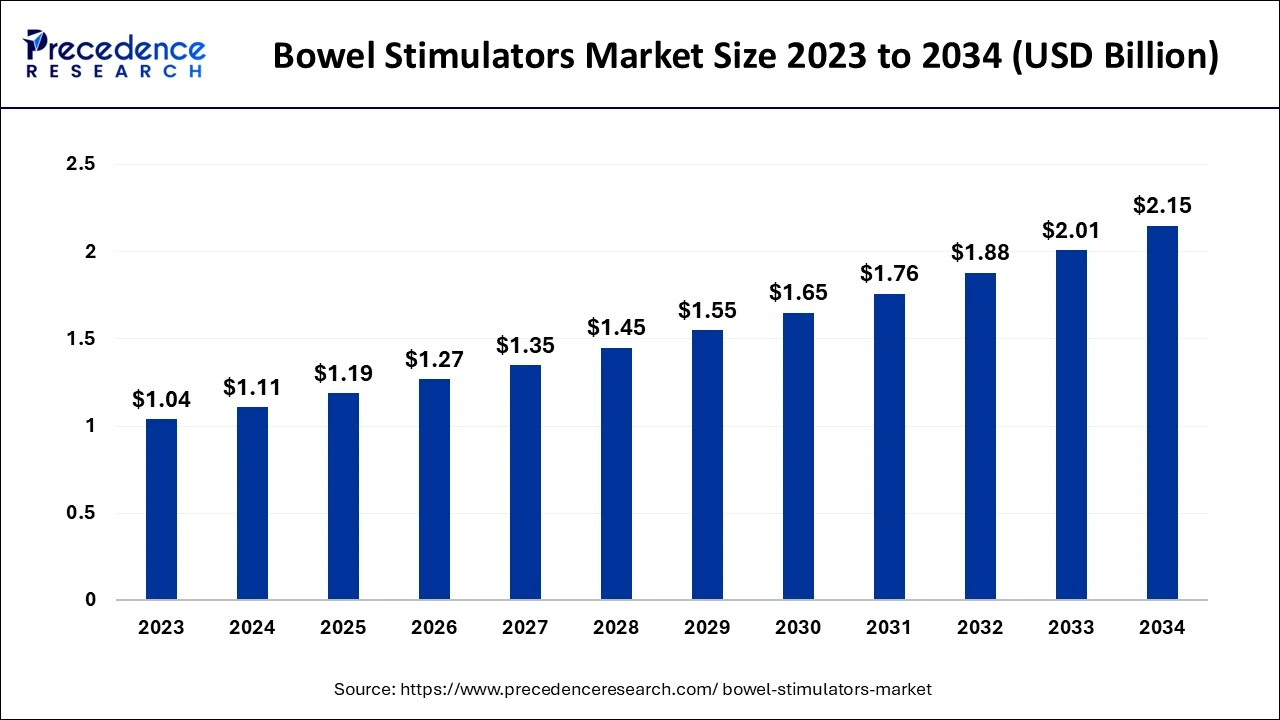
The global bowel stimulators market size was estimated at USD 1.11 billion in 2024 and is predicted to increase from USD 1.19 billion in 2025 to approximately USD 2.15 billion by 2034, expanding at a CAGR of 6.83% from 2025 to 2034. The bowel stimulators market is driven by the growing aging population
Bowel Stimulators Market Key Takeaways
- In terms of revenue, the global bowel stimulators market was valued at USD 1.11 billion in 2024.
- It is projected to reach USD 2.15 billion by 2034.
- The market is expected to grow at a CAGR of 6.83% from 2025 to 2034.
- North America dominated the bowel stimulators market in 2024.
- Asia-Pacific is observed to be the fastest-growing in market during the forecast period.
- By product type, the external segment accounted for the largest market share of 58% in 2024.
- By product type, the implantable segment shows significant growth in the market during the forecast period.
- By application, the urinary and fecal incontinence segment contributed the highest market share of 54% in 2024.
- By end-user, the hospitals and clinics segment dominated the bowel stimulators market in 2024.
How is AI helping the Bowel Stimulators Market Growth?
Artificial Intelligence (AI) algorithms can examine patient data, such as medical history and prior treatment reactions, to develop customized bowel stimulation protocols. Patient happiness and treatment effectiveness are improved by this customization. AI can optimize the design of intestinal stimulators during the development stage by testing different configurations and settings through simulations. This improves device performance while cutting expenses and development time. Artificial intelligence can improve the planning and implementation of clinical trials for novel bowel stimulators by identifying appropriate patient populations, trial protocol optimization, and more effective result analysis.
Market Overview
Effective treatment options are becoming increasingly important due to the rising prevalence of gastrointestinal (GI) problems, including irritable bowel syndrome (IBS), fecal incontinence, and persistent constipation. Patients who do not react to conventional therapy may find relief using bowel stimulators. By dramatically enhancing gastrointestinal function, bowel stimulators can improve patient outcomes, quality of life, and reliance on potentially harmful drugs. Increased patient satisfaction and treatment compliance may follow from this.
- In October 2023, Teva Pharmaceutical Industries will work with French drugmaker Sanofi and open a new tab to develop a treatment for inflammatory bowel disease.
- In February 2022, Medtronic plc, a global leader in healthcare technology, disclosed it received approval from the U.S. Food and Drug Administration (FDA) for Inter Stim X. These systems are the standard of care in advanced therapy options and the most personalized system to deliver sacral neuromodulation (SNM) therapy.
Bowel Stimulators Market Growth Factors
- Bowel incontinence, a disorder that impairs the capacity to control bowel movements, is becoming more common as the world's population ages. This directly impacts the need for bowel stimulators as a therapeutic alternative.
- Bowel stimulators are covered by health insurance plans in many nations, increasing patient access and propelling market expansion.
- Bowel stimulators have historically been used mostly to treat fecal incontinence, but they are now also being utilized to treat persistent constipation and neurogenic bowel dysfunction. The market is growing due to this expansion of the target patient base.
- Bowel stimulators are becoming a more popular treatment choice among patients and medical professionals as more people learn about their advantages and efficacy.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 2.15 Billion |
| Market Size in 2024 | USD 1.11 Billion |
| Market Size in 2025 | USD 1.19 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.83% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product Type, Application, End-user, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East, & Africa |
Market Dynamics
Driver
Increasing Prevalence of Bowel Incontinence
Patients are more likely to seek therapy now that non-surgical and less invasive surgical treatments are available. When compared to conventional surgical techniques, bowel stimulators can frequently be installed with less recovery time. Better diagnostic and treatment recommendations result from improved education for healthcare professionals on bowel management and the available devices, increasing the market for bowel stimulators.
- In October 2023, the U.S. Food and Drug Administration (FDA) approved mirikizumab, a highly effective new treatment for ulcerative colitis (UC). This therapy offers a safe, effective treatment option for patients. Unlike existing treatments for UC, it also relieves a key symptom, bowel urgency, significantly impacting patients' lifestyle.
- In 2023, as detailed in our annual New Drug Therapy Approvals report, CDER approved 55 novel drugs, expanding some previously approved therapies' indications or patient populations.
Restraint
Taboos Associated with Fecal Disorders
There is frequently shame and embarrassment associated with fecal problems, which include ailments including constipation, fecal incontinence, and other bowel irregularities. The public is less aware of treatment choices like bowel stimulators since many people shy away from talking about these problems out of fear of being judged. Some people may be deterred from considering these choices because they are worried about the possible adverse effects of bowel stimulators or because they are afraid of becoming reliant on them for regular bowel movements.
Opportunity
Advancements in Technology
Smaller, more effective bowel stimulators have been created due to materials science and microelectronics developments. These little devices increase patient comfort and compliance and can be worn externally or implanted. Improvements in technology frequently result in quicker and more effective regulatory approval procedures. Innovative technology-using devices might be given preference, enabling businesses to launch their goods faster. The likelihood of negative responses and complications related to implanted devices is decreased by using biocompatible materials. Hydrogels and bioresorbable polymers are examples of materials science innovations that help create safer gadgets.
Product Type Insights
The external segment dominated the bowel stimulators market in 2024. The creation of sophisticated external intestinal stimulators has improved patient comfort, usability, and efficacy. Innovations like noninvasive stimulation treatments have increased patient compliance. In individuals with neurological illnesses, external bowel stimulators can help with a variety of bowel-related problems, such as constipation or bowel control. Their adaptability raises their market reach.
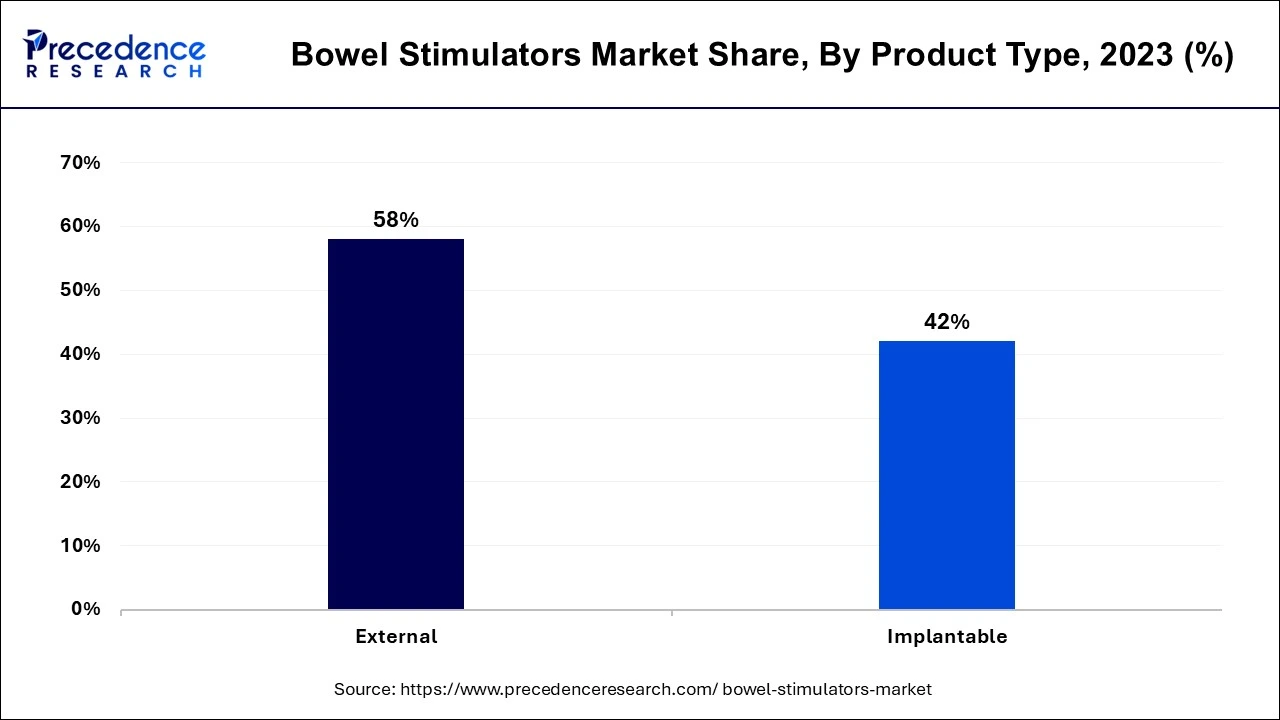
The implantable segment shows significant growth in the bowel stimulators market during the forecast period. Patient outcomes have improved due to the introduction of sophisticated implantable bowel stimulators that employ neuromodulation techniques. These devices can efficiently regulate bowel motions by stimulating nerves, providing a non-pharmaceutical option for treating diseases, including persistent constipation and fecal incontinence. Manufacturers enter into strategic alliances with medical facilities and academic institutions to enhance clinical research and product development. This partnership may result in creative answers to unmet gastrointestinal stimulation requirements.
- In November 2023, Medtronic plc, a global leader in healthcare technology, declared that the U. S. Food and Drug Administration (FDA) has approved the Symplicity Spyral renal denervation (RDN) system for treating hypertension. With this approval, Medtronic will immediately begin commercialization.
Application Insights
The urinary & fecal incontinence segment dominated the bowel stimulators market in 2024. The market for bowel stimulators has seen technological developments that increase therapeutic effectiveness. Patients have more individualized and efficient management options due to devices with biofeedback mechanisms and stimulation technology. Bowel stimulators are being suggested by medical professionals more frequently as a component of all-encompassing therapy programs for people suffering from fecal and urine incontinence. A greater understanding of their efficacy in symptom management and enhancing patients' quality of life is reflected in this integration.
- In October 2023, Eli Lilly and Co. stated that the U.S. health regulator had approved its drug for treating adults with moderate-to-severe active ulcerative colitis, a type of chronic inflammatory bowel disease. The drug, available in the United States under the brand name Omvoh, alongside tirzepatide for obesity, pirtobrutinib for cancer, and lebrikizumab for atopic dermatitis or eczema.
End-user Insights
The hospitals and clinics segment dominated the bowel stimulators market in 2024. As the population ages and lifestyles change, gastrointestinal diseases like bowel blockage, constipation, and irritable bowel syndrome (IBS) are becoming more prevalent. Patients seek diagnosis and treatment for these illnesses, primarily in hospitals and clinics. Clinics and hospitals often participate in research studies and clinical trials that confirm the effectiveness of bowel stimulators. These studies raise awareness and motivate medical professionals to use these treatments as part of routine treatment plans.
Regional Insights
North America dominated the bowel stimulators market in 2023. Gastrointestinal conditions, including constipation, irritable bowel syndrome (IBS), and other motility problems, affect a sizable section of the population. Effective bowel stimulators are in high demand due to this high frequency. Innovative bowel stimulation devices can now be approved more quickly because of rules set by regulatory bodies like the U.S. Food and Drug Administration (FDA). This incentivizes businesses to launch new goods.
- According to the American College of Gastroenterology, more than 5.5 million Americans have fecal incontinence. It is more common in older people and women.
Asia- Pacific is observed to be the fastest growing in the bowel stimulators market during the forecast period. The availability and efficacy of bowel stimulators are becoming more widely known to patients and healthcare professionals. Innovative medical gadgets are being accepted at higher rates due to patients' increased awareness of their treatment options. Healthcare organizations' campaigns and educational programs have also influenced this trend to raise awareness about intestinal health. The market for bowel stimulators is expanding due in large part to the demographic trend toward an elderly population in nations like South Korea, China, and Japan. The need for efficient gut health management is growing along with the number of seniors.
- In December 2022, Biocon Limited declared initiating the clinical study of Itolizumab, a monoclonal antibody for ulcerative colitis (UC) in patients with UC in India, in collaboration with Equillium Inc.
Bowel Stimulators Market Companies

- Medtronic
- 3M
- Coloplast A/S
- B. Braun
- Axonics Inc.
- Laborie Inc.
- Cyberonics
- Sportaid
- Nuvectra
- Cogentix Medical
Recent Developments
- In February 2022, the newest version of the InterStim portfolio's recharge-free device, InterStim X, was approved by the U.S. Food and Drug Administration (FDA) and is now accessible, according to Medtronic plc.
Segments Covered in the Report
By Product Type
- External
- Implantable
By Application
- Urinary & Fecal Incontinence
- Chronic Anal Fissure
- Others
By End-user
- Hospitals And Clinics
- Ambulatory Surgical Centers
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting