What is the eClinical Solutions Market Size?
The global eClinical solutions market size is calculated at USD 12.82 billion in 2025 and is predicted to increase from USD 14.28 billion in 2026 to approximately USD 27.14 billion by 2035, expanding at a CAGR of 7.79% between 2026 and 2035.
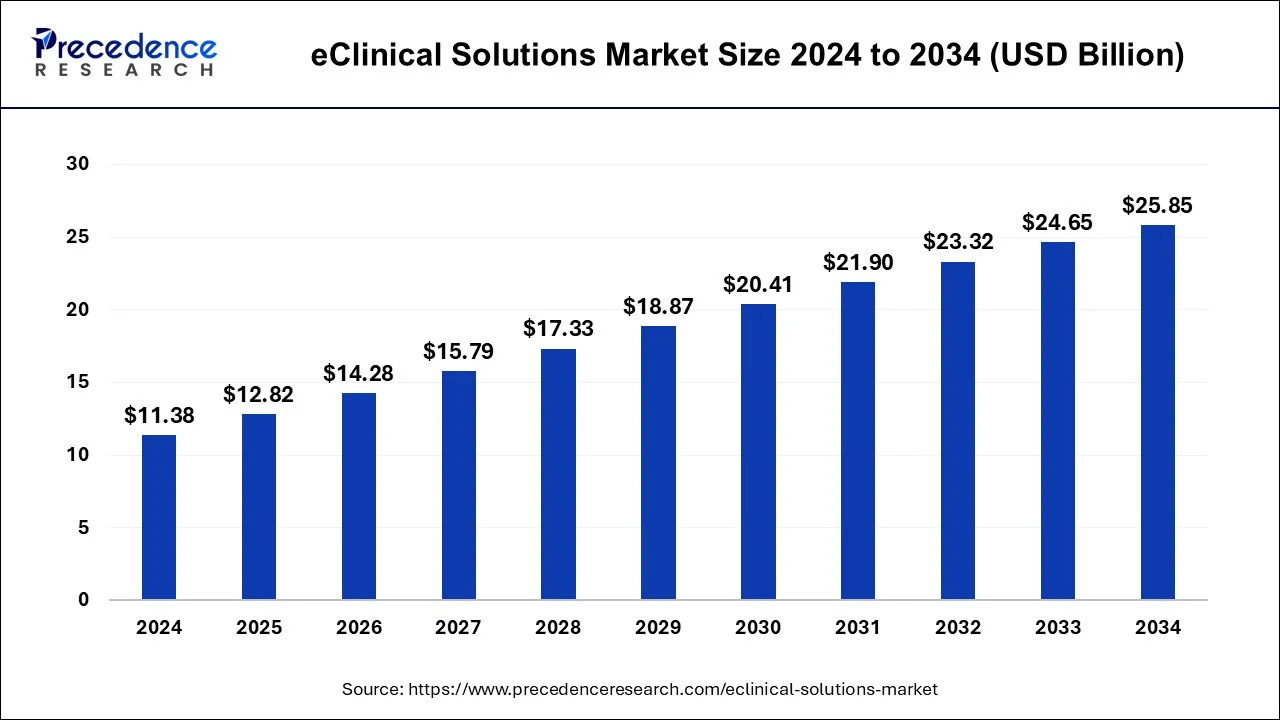
eClinical Solutions Market Key Takeaways
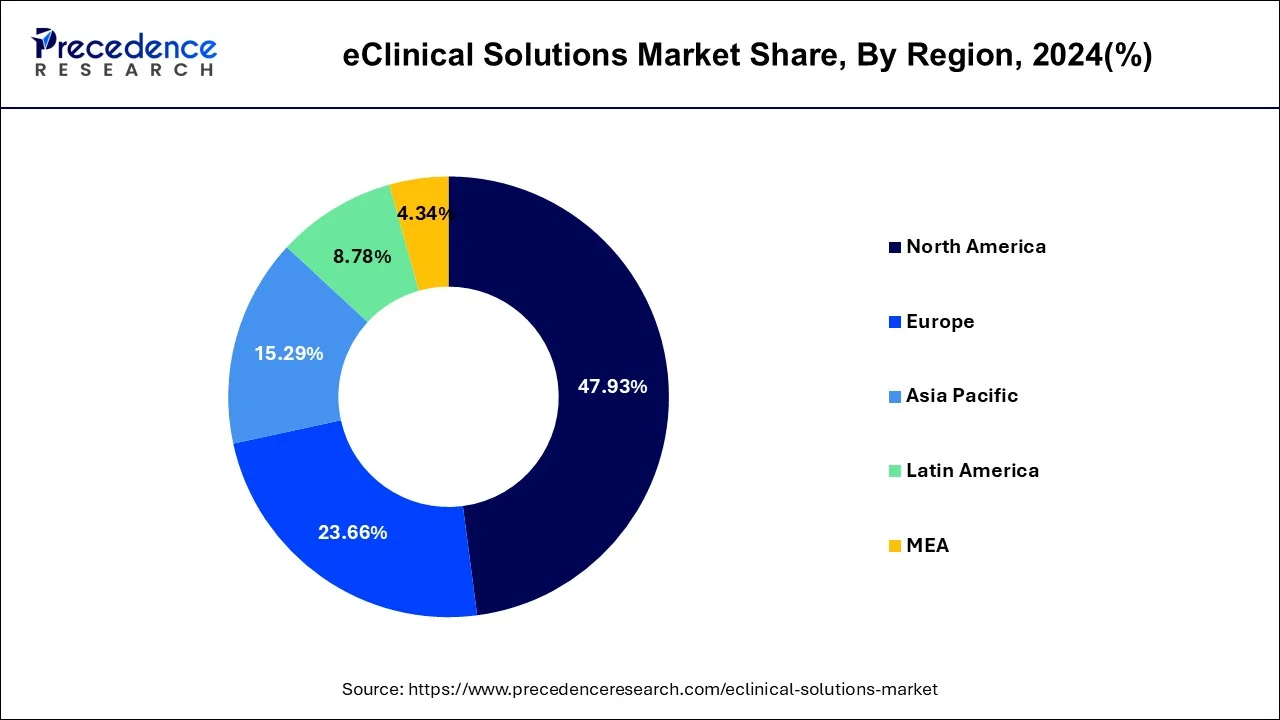
- North America led the eClinical solutions market with the largest market share of 47.93% in 2025.
- By solution type, the electronic data capture (EDC) segment held a 17.80% market share in 2025.
- By solution type, the Artificial Intelligence/Machine Learning in eClinical segment is expected to grow at the highest CAGR of 15.40% over the forecast period.
- By delivery mode, the web-based / cloud-based solutions segment led the market with 61.20% share in 2025 and is expected to grow at the fastest CAGR of 12.60% over the forecast period.
- By clinical trial phase, the phase III segment led the market by holding 42.50% market share in 2025.
- By clinical trial phase, the phase IV / post-marketing surveillance segment is expected to grow at the highest CAGR of 11.30% over the forecast period.
- By end user, the pharmaceutical & biopharmaceutical companies segment held a 36.70% market share in 2025.
- By end user, the contract research organizations (CROs) segment is expected to grow at the highest CAGR of 13.10% during the projected period.
- By therapeutic area, the oncology segment dominated the market with 31.60% market share in 2025.
- By therapeutic area, the rare diseases / orphan drugs segment is expected to grow at the highest CAGR of 14.70% over the forecast period.
Digitizing Clinical Trials: How e-clinical Solution Are Transforming Drug Development
The e-clinical solutions market brings digital transformation to clinical development by digitizing trial design, patient recruitment, data capture, monitoring, and regulatory submissions. E-clinical covers eCRF/eTRF electronic case report/targeted report forms, eConsent, ePRO/eCOA electronic patient-reported outcomes / clinical outcome assessments, clinical trial management systems (CTMS), randomisation and trial supply management RTSM/IWRS, remote monitoring tools, safety/pharmacovigilance platforms, electronic trial master file (eTMF) systems, and integrated data warehouses for analytics and real-world evidence.
The market's value proposition is faster study start-up, better data quality, streamlined regulatory compliance, improved patient engagement, and lower overall operational costs through automation and centralized data flows.
eClinical Solutions Market Growth Factors
Integration of software solutions in clinical trials provides tremendous market growth. Further, rising trend of clinical trial outsourcing services to Contract Research Organizations (CROs) coupled with rising number of life sciences organizations and CROs poised to help the market to gain significant traction over the forecast period. Increasing research activities in Asian countries for the development of cost-effective solutions or modules expected to spur the market growth.
Presence of strict regulations for clinical trial together with rising need for safety monitoring plays a vital role in the fuelling adoption of eClinical solutions especially in the developed countries such as the United States. For instance, the National Institutes of Health and the U.S. Department of Health and Human Services have issued stringent regulations on clinical trial registration requirements; additionally they promote clinical data sharing.
Biopharma and pharma companies seeks surge in demand of software solutions for clinical trials that accounts as one of the primary factor to stimulate the overall market growth. Apart from this, favorable government policies to expand the end-users of eClinical solutions as well as offer grants to substantiate trials are likely to propel the market growth during the analysis period.
Market trends
- In December 2024, a collaboration between eClinical Solutions LLC, which is a provider of digital clinical software and services, and Snowflake, which is an AI Data Cloud Company, was announced. To develop modern clinical data architecture as well as to address growing data challenges by life sciences organizations, a bidirectional integration will be developed by this collaboration. Furthermore, the Snowflake AI Data Cloud is completely merged with eClinical's Elluminate Clinical Data Cloud for this collaboration. (Source: https://finance.yahoo.com)
- In November 2024, a novel Randomization and Trial Supply Management (“RTSM”) platform, which will significantly drive clinical development technology, that is ClinPhone 5, was launched and announced by Perceptive eClinical, which is a part of Perceptive Group. To provide greater efficiencies to Perceptive customers, this platform will hasten the development of treatments, life-saving drugs, as well as innovation, which in turn, will improve the user experience, which is the main goal of this collaboration. (Source: https://www.pharmaceutical-technology.com)
Market Outlook
- Industry Overview: The e-clinical industry comprises software vendors (SaaS and enterprise), CROs with embedded digital capabilities, integrators and middleware providers, device and mobile app developers, data-standards organizations, and specialist consultancies for validation and regulatory compliance. Vendors supply modular or suite-level offerings that map to specific trial functions (CTMS, eTMF, eConsent, ePRO) while larger platforms aim to provide end-to-end orchestration.
- CROs and large pharma are increasingly building hybrid operating models that combine in-house digital teams with vendor partnerships. Key capabilities include validated systems for regulated environments, robust audit trails, user training and change management, API-driven interoperability with EHRs and labs, and advanced analytics for trial optimization and adaptive designs.
- Sustainability Trend: Sustainability for e-clinical solutions spans environmental, operational, and social dimensions. Environmentally, virtualization and cloud-native architectures reduce the need for physical trial footprint (less travel, fewer site visits), lowering the carbon footprint of clinical development. Operational sustainability focuses on efficiency, reducing redundant data entry, automating repetitive workflows, and shortening study timelines to conserve financial and human resources.
- Social sustainability manifests as broader patient inclusion via remote visits and decentralized trials, improving access for underserved or geographically dispersed populations. Responsible data governance and inclusive design practices (multi-language eConsent, accessible ePRO interfaces) are also central to sustainable e-clinical ecosystems.
- Major Investment Themes: Investment is concentrated on platform consolidation single vendors that can orchestrate multiple trial functions, decentralized trial enablement, telemedicine integrations, wearables, remote monitoring, AI/ML for site selection, risk-based monitoring, predictive enrollment, and interoperability standards that reduce integration friction.
- Other investment themes include validated privacy-preserving analytics, federated learning for cross-sponsor insights, real-world data integration for hybrid evidence generation, automation of regulatory submission packages, and solutions that reduce administrative burden, automated source data verification, and auto-populating eTMF. Investors also target vertical specialization, e.g., oncology or rare disease trial stacks that handle complex endpoints and registries.
- Sustainable Ecosystems and Startups: Sustainable e-clinical ecosystems emerge when technology vendors, CROs, patient advocacy groups, and health systems co-create validated workflows and shared data standards. Startups often focus on a single pain point, eConsent UX, remote vitals capture, decentralized site orchestration, patient retention platforms, and then integrate into broader stacks via APIs.
- Accelerators and consortia that promote open standards, shared testbeds, and interoperability pilots help small innovators scale without re-inventing regulatory or compliance frameworks. Successful startup strategies include partnering with CROs for real trials, designing for regulatory validation from day one, and prioritizing low-bandwidth, privacy-first solutions that work across regions.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2026 to 2035 | CAGR of 7.79% |
| Market Size in 2025 | USD 12.82 Billion |
| Market Size in 2026 | USD 14.28 Billion |
| Market Size by 2035 | USD 27.14 Billion |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | By Product, By Development Phase, By Delivery Mode and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Enormous demand for real-time data access
The advancements in the healthcare sector across the globe have forced providers to adopt remote monitoring, real-time data access supports remote monitoring of patients participating in clinical trials. With the use of eClinical solutions, patients can report data remotely through electronic patient-reported outcome tools or wearable devices. Real-time data access enables healthcare professionals, researchers, and sponsors to make informed decisions promptly. With eClinical solutions, data collected during a clinical trial is instantly available, allowing stakeholders to monitor patient safety, track study progress, and make critical decisions in real-time. This facilitates faster intervention, adjustment of trial protocols, and immediate response to safety concerns or adverse events. Considering the factors that offer real-time data with eClinical solutions, the element is observed to act as a driver for the market.
Rising number of clinical trials
With the increasing number of clinical trials, collaboration among various stakeholders becomes crucial. eClinical solutions facilitate seamless communication and collaboration between sponsors, investigators, CROs, regulatory authorities, and other involved parties. These solutions provide centralized platforms for real-time data sharing, document exchange, and remote monitoring. Enhanced collaboration accelerates decision-making, promotes transparency, and ensures effective coordination among trial participants. The growing number of clinical trials generates vast amounts of data that require analysis and reporting. eClinical solutions provide advanced analytics capabilities to extract valuable insights from the collected data. These solutions offer tools for data visualization, statistical analysis, and generation of reports, enabling researchers and trial sponsors to make informed decisions and communicate trial outcomes effectively. Thus, the requirements for data analysis along cost efficiency offered by eClinical solutions for clinical trials makes it a driving factor for the market.
Restraint
Risk of errors
Errors can occur during the data entry process, leading to inaccuracies and inconsistencies in the data. Human errors, such as typos, incorrect data input, or data mapping mistakes, can negatively impact the integrity and reliability of the collected data. Even small errors in data entry can have significant consequences, affecting the outcomes of clinical trials and potentially compromising patient safety. Moreover, ensuring compliance can be challenging, and non-compliance can lead to penalties, legal issues, or data integrity concerns, posing a restraint to the adoption of eClinical solutions.
Lack of standardization
In clinical trials, data is generated from multiple sources, including different sites, investigators, devices, and systems. Without standardized data models and formats, the process of integrating and harmonizing data becomes complex and time-consuming. This lack of standardization can result in data inconsistencies, errors, and difficulties in performing cross-study analysis, meta-analysis, or comparative effectiveness research. In the absence of standardized data collection methods and formats, there is a higher risk of data quality issues, including errors, inconsistencies, and missing data. This lack of standardization can impact the reliability and integrity of clinical trial data, potentially leading to flawed analysis, biased results, or inaccurate conclusions. Thus, lack of standardization creates a restraint for the market.
Opportunity
Technological advancements
The integration of big data analytics and eClinical solutions allows for in-depth analysis of vast amounts of clinical and non-clinical data. It enables the identification of novel biomarkers, identification of patient subgroups, optimization of trial design, and predictive modeling. These insights can lead to more personalized and targeted interventions, improved trial outcomes, and enhanced patient care. Advanced communication and collaboration tools facilitate seamless interactions among study teams, investigators, and sponsors. Virtual trial platforms enable decentralized or hybrid trial models, eliminating the need for physical site visits and reducing logistical challenges. These technologies enable broader participant recruitment, faster trial execution, and cost savings; all these are observed to offer technological advancements in the market.
Global expansion of clinical services
The use of real-world data in clinical research is expanding, enabling the evaluation of treatment outcomes and safety profiles in real-life settings. The global expansion of clinical services necessitates collaboration among various stakeholders, including pharmaceutical companies, CROs, research institutions, regulatory bodies, and technology providers. eClinical solutions offer platforms and tools that enable seamless collaboration, data sharing, and secure communication among these entities. The opportunity lies in fostering partnerships and alliances to deliver integrated eClinical solutions that meet the specific needs of global clinical trials and research endeavors.
Challenge
Complexities in adoption in underdeveloped areas
Underdeveloped areas may have limited access to modern technology and digital devices. Clinical research sites, healthcare facilities, and research organizations in these areas may not have the necessary resources to invest in eClinical solutions or update their existing technology infrastructure. The lack of access to computers, tablets, or smartphones can impede the adoption of eClinical solutions, as these tools are essential for data capture, entry, and communication. Thus, complicated adoption in underdeveloped areas is observed to create a challenge for the market.
Segment Insights
Solution Type Insights
The electronic data capture (EDC) segment held a 17.80% market share in 2025. The strong emphasis on optimizing clinical workflows has boosted the adoption of electronic data capturing (EDC) solutions. These solutions allow quicker and more efficient data entry by reducing human errors. In addition, EDC solutions reduce the time required for data processing and analysis, accelerating clinical trials. The rise in popularity of remote clinical trials further bolsters segmental growth.
The Artificial Intelligence/Machine Learning in eClinical segment is expected to grow at the highest CAGR of 15.40% over the forecast period. The growth of the segment can be credited to the increasing demand for predictive analytics, along with the innovations in computational power and extensive data availability. Recent developments in AI and ML have brought significant strides in identifying and predicting health emergencies and disease populations.
Delivery Mode Insights
The web-based / cloud-based solutions segment led the market with 61.20% share in 2025 and is expected to grow at the fastest CAGR of 12.60% over the forecast period. The dominance and the growth of the segment are mainly due to its associated benefits that include usability, easy accessibility, and lower investments required. Further, the web-hosted solutions can be easily customized, so providers can customize their offering as per the user group. Further, these solutions have a higher level of interoperability. The aforementioned factors help the segment to maintain its position during the forecast period.
eClinical Solutions Market Revenue (USD Million), By Delivery Mode, 2022-2024
| Delivery Mode | 2022 | 2023 | 2024 |
| Licensed enterprise (on-premise) Solutions | 1,060.8 | 1,206.7 | 721.1 |
| Cloud-based (SAAS) Soutions | 3,211.6 | 3,698.7 | 2,213.9 |
| Web-hosted (On-Demand) Solutions | 4,457.7 | 5,098.9 | 2,993.3 |
End-use Insights
The pharmaceutical & biopharmaceutical companies segment held a 36.70% market share in 2024. The eClinical solutions not only enhance the efficiency of the trial but also reduce the time and cost required during drug development. This time and money can be leveraged by companies to speed up the production process, driving market expansion further.
The contract research organizations (CROs) segment is expected to grow at the highest CAGR of 13.10% during the projected period. This is attributed to the rising concern of pharmaceutical companies to reduce their overall expenditure. Moreover, the increasing application of eClinical solutions in research activities has further broadened the scope of the segment. Benefits of outsourcing clinical trials to CROs comprise enhanced productivity, increased efficiency of services, cost advantages, and increased focus on core areas of development that are critical for a company's growth.
eClinical Solutions Market Revenue (USD Million), By End-use, 2022-2024
| End-use | 2022 | 2023 | 2024 |
| Contract Research Organizations (CROs) | 3,446.6 | 3,963.9 | 2,334.7 |
| Medical Device Companies | 1,511.6 | 1,726.0 | 1,030.4 |
| Pharma/Biotech Companies | 2,272.5 | 2,599.5 | 1,526.3 |
| Hospitals & Clinics | 1,030.7 | 1,183.0 | 713.5 |
| Others | 468.8 | 531.9 | 323.3 |
Therapeutic Area Insights
The oncology segment dominated the market with a 31.60% market share in 2025. The dominance of the segment is owing to the increasing prevalence of cancer across the globe, coupled with the technological advancements in drug development. Furthermore, significant investments by pharmaceutical companies and governments, organizations can impact segment growth soon.
The rare diseases / orphan drugs segment is expected to grow at the highest CAGR of 14.70% over the forecast period. The growth of the segment can be linked to the rising incidence of rare diseases and innovations in biotechnology, genomics, and precision medicine. Raised medical public awareness regarding rare diseases and their impact boosts the demand for effective treatment alternatives.
Therapeutic Area Insights
The oncology segment dominated the market with a 31.60% market share in 2025. The dominance of the segment is owing to the increasing prevalence of cancer across the globe, coupled with the technological advancements in drug development. Furthermore, significant investments by pharmaceutical companies and governments, organizations can impact segment growth soon.
The rare diseases / orphan drugs segment is expected to grow at the highest CAGR of 14.70% over the forecast period. The growth of the segment can be linked to the rising incidence of rare diseases and innovations in biotechnology, genomics, and precision medicine. Raised medical public awareness regarding rare diseases and their impact boosts the demand for effective treatment alternatives.
Regional Insights
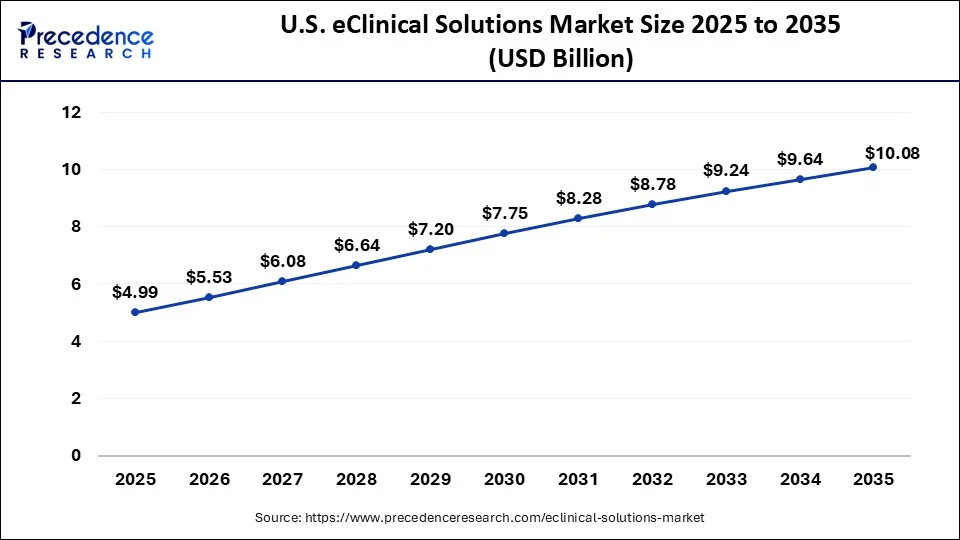
What is the U.S. eClinical Solutions Market Size?
The U.S. eClinical solutions market size accounted for USD 4.99 billion in 2025 and is expected to be worth around USD 10.08 billion by 2035, poised to grow at a CAGR of 7.28% from 2026 to 2035.
North America Emerges While Asia Pacific Emerges as a High-Growth Hub for eClinical Solutions
North America led the global eClinical solutions market in terms of revenue in the year 2025. Rising prevalence of lifestyle accompanied with several diseases such as cardiac disorder and diabetes along with increasing number of target population are the key factors that poised to stimulate the demand of eClinical solutions in the region. Apart from this, the Asia Pacific exhibits lucrative growth over the analysis period owing to rising prevalence of chronic diseases that include cardiovascular conditions, cancer, and infectious diseases. Furthermore, many Asian countries such as India, China, Japan, and Korea outsources large number of clinical trials due to large patients population in the countries, thereby boosting the adoption of eClinical solutions in above mentioned regions.
Europe Gains Momentum Through Digital Clinical Trials and Supportive Regulations
Europe is expected to grow significantly in the eClinical solutions market during the forecast period. Growing digitalization, along with the clinical trials in Europe, is increasing the demand for eClinical solutions. Furthermore, the investments, as well as the rules and regulations provided by the government and regulatory bodies, respectively, are encouraging their safe and reliable use. Thus, this promotes the market growth.
UK eClinical Solutions Market Trends:
The adoption of advanced technologies in various industries in the UK, as well as the growing collaborations with pharma industries and AI companies, is accelerating the development and the various features of eClinical solutions, due to their growing use. Moreover, their compliance with the regulations laid by the regulatory bodies helps in enhancing the safety of the process.
Germany eClinical Solutions Market Trends:
The use of eClinical solutions for improving the patient's compliance and communication, with reduced cost, is increasing in Germany. Thus, with the help of advanced technologies, their development is rising. These developments are further supported by the government investments.
Can Latin America Leapfrog Clinical Barriers with Digital Trials?
Latin America presents a compelling mix of high patient pools, growing clinical trial activity, and variable digital infrastructure. Countries like Brazil, Mexico, Chile, and Argentina host many regional studies and are attractive for enrolment, especially for diverse patient representation. eClinical adoption is often hybrid: centralized eCRF and CTMS systems are common for multinational studies, while remote monitoring and eConsent uptake depend on internet penetration and local regulatory clarity. Language localization, culturally appropriate patient engagement tools, and low-bandwidth designs are key to success.
Opportunities include partnering with regional CROs to run decentralized elements, local phlebotomy, home nursing, and leveraging mobile-first ePRO tools to improve retention. Barriers include heterogeneous regulatory landscapes, data-residency concerns, and limited local experience with full decentralized trial governance issues that can be mitigated via staged pilots and strong local partnerships.
Brazil Healthcare Cloud Computing Market Trends
Brazil's market is growing due to increasing clinical trial activity, rising adoption of digital technologies, and a large patient population supporting study recruitment. Expanding outsourcing of trials, growing presence of CROs, and regulatory efforts to modernize clinical research are driving demand for eClinical platforms that improve data management, compliance, and operational efficiency.
How is the e-Clinical Exploring the Solutions Market in Asia-Pacific?
The Asia-Pacific region is witnessing a significant transformation in the e-clinical solutions market, driven by rapid technological advancements and an increasing focus on enhancing clinical trial efficiency. This growth is particularly notable in India, where a robust healthcare infrastructure, a burgeoning technology sector, and a large patient population converge to create a fertile ground for e-clinical solutions.
India e-Clinical Solutions Market Trends
India stands out in the Asia-Pacific e-clinical solutions market due to its unique combination of factors. The country's large and diverse patient population offers a rich source for clinical trials. Moreover, India's thriving IT industry, combined with a growing focus on healthcare innovation, supports the development of cutting-edge e-clinical technologies. Key players in the Indian market are exploring collaborations with global counterparts to enhance their service offerings and ensure compliance with international standards.
The government's initiatives to modernize healthcare systems, align with global standards, and promote research and development are further propelling the growth of e-clinical solutions. Moreover, the increasing importance of real-world evidence and patient-centric trial designs aligns well with India's capabilities.
Middle East and Africa Accelerate Adoption of eClinical Solutions
The Middle East and Africa market is expanding due to growing clinical research activity, increasing adoption of digital health technologies, and investments in healthcare infrastructure. Rising prevalence of chronic diseases, improving regulatory frameworks, and greater outsourcing of clinical trials are driving demand for eClinical platforms that enhance data accuracy, compliance, and operational efficiency across the region.
UAE Healthcare Cloud Computing Market Trends
The UAE market is increasing due to strong government support for healthcare digitalization, rising clinical research activity, and growing adoption of advanced data management technologies. Expansion of medical research centers, focus on regulatory compliance, and increasing use of remote and decentralized clinical trial models are further driving demand for eClinical platforms across the country.
Market Value Chain Analysis
- Raw Material Sourcing: In software-centric markets, raw materials are code libraries, validated cloud infrastructure, device integrations, secure identity and consent frameworks, standard clinical datasets (CDISC, HL7/FHIR mappings), and trained personnel (clinical data managers, validation engineers, regulatory affairs specialists).
- Technological Advancements: Technological progress shaping e-clinical solutions includes APIs and middleware that enable seamless EHR and lab integrations; wearable sensors and IoT devices that generate continuous, objective endpoints; eConsent platforms with multimedia and dynamic consent capabilities.
Key Companies and Market Share Insights
The global eClinical solutions market encounters huge competition among the market players owing to a race among players to capture the untapped market opportunity. These players adopt various growth strategies that are mergers & acquisitions, new product development, and collaborations that help them to consolidate their position in the market. For example, in September 2019, Anju Software Inc. acquired OmniComm Systems to enrich its portfolio of eClinical solutions with innovative data analytical capabilities.
eClinical Solutions Market Companies
- eClinicalWorks: A leading provider of cloud-based electronic health record (EHR) and practice management solutions for ambulatory medical practices. Its importance lies in streamlining clinical workflows, managing patient data, and facilitating communication between healthcare providers. By digitizing health records, eClinicalWorks enables a more efficient and data-driven approach to patient care, supporting the underlying data needs of precision medicine initiatives at the point of care.
- OmniComm Systems:Its flagship product, TrialMaster, is an electronic data capture (EDC) system used in clinical trials. OmniComm's importance is in ensuring the efficient, accurate, and compliant collection and management of clinical trial data, which is crucial for the research and development of new precision medicine drugs and diagnostics.
- IBM Watson Health:While parts of the business have been acquired by other entities like Francisco Partners, now operating as Merative, the core idea demonstrated the potential of AI in accelerating drug discovery, personalizing treatment plans, and improving health outcomes.
- Medidata: Provides cloud-based eClinical platforms, including EDC, eConsent, ePRO, CTMS, RTSM, and analytics solutions to improve clinical trial efficiency, data quality, and regulatory compliance.
- Veeva Systems: Offers integrated eClinical, CTMS, eTMF, EDC, and regulatory solutions on a unified cloud platform, enabling faster trial execution, better data visibility, and streamlined compliance.
- IQVIA: Delivers end-to-end eClinical solutions combining data management, analytics, real-world evidence, and decentralized trial technologies to optimize clinical development and decision-making.
- Oracle: Provides comprehensive eClinical solutions, including EDC, CTMS, RTSM, safety, and analytics tools designed to support global clinical trials with secure data management and compliance.
- Parexel International: Offers clinical research services and eClinical technologies supporting trial design, data capture, regulatory submissions, and patient engagement across multiple therapeutic areas.
- Signant Health: Specializes in digital trial solutions such as eCOA, RTSM, eConsent, and patient engagement technologies to improve data integrity and trial outcomes.
- Clario: Provides endpoint technology solutions, including imaging, cardiac safety, respiratory analytics, and eCOA platforms, to deliver high-quality, centralized clinical trial data.
Recent Development
- On 14th Nov 2024, Perceptive e-clinic launched ClinPhone 5. A partnership with CRScube, a South Korean clinical research technology, leveraged the development of life-saving drugs, treatments, and innovations to perceptive customers.
- On 18th Dec 2024, E-clinical solutions and Snowflake integrated clinical data management. A partnership with a strategic vision to establish a bidirectional integration to address challenges results in effective and efficient growth.
- On 1st May 2024, Oracle advanced global RTSM capabilities to help sponsors. Engaging with sponsors enhances the usage, access. Also, empowers the ability to automate creation, modify, and create rules.
Segments Covered in the Report
By Solution Type
- Electronic Data Capture (EDC)
- Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Randomization and Trial Supply Management (RTSM)
- Electronic Patient Reported Outcomes (ePRO)
- Electronic Clinical Outcome Assessment (eCOA)
- Clinical Analytics Platforms
- Clinical Data Integration Platforms
- eConsent
- Safety Solutions (pharmacovigilance platforms)
- Regulatory Information Management (RIM)
- Risk-Based Monitoring (RBM)
- Medical Imaging Solutions
- Electronic Trial Master File (eTMF)
- Wearable/Remote Monitoring Devices Integration
- Artificial Intelligence/Machine Learning in eClinical
By Development Phase
- Phase IV
- Phase III
- Phase II
- Phase I
By Delivery Mode
- Web-based / Cloud-based Solutions
- On-Premise Solutions
- Hybrid Deployment Models
By Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV / Post-Marketing Surveillance
By End-User
- Pharmaceutical and Biopharmaceutical Companies
- Contract Research Organizations (CROs)
- Academic Research Institutions
- Hospitals and Clinics
- Medical Device Companies
- Regulatory Bodies and Government Research Agencies
By Therapeutic Area
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Immunology
- Respiratory
- Endocrinology (e.g., Diabetes)
- Rare Diseases / Orphan Drugs
- Gastroenterology
- Pain Management
- Dermatology
- Other Emerging Therapeutic Area
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content



