What is the Lyme Disease Diagnostic Devices Market Size?
The global lyme disease diagnostic devices market size is accounted at USD 2.82 billion in 2025 and predicted to increase from USD 2.97 billion in 2026 to approximately USD 4.79 billion by 2035, expanding at a CAGR of 5.44% from 2026 to 2035.
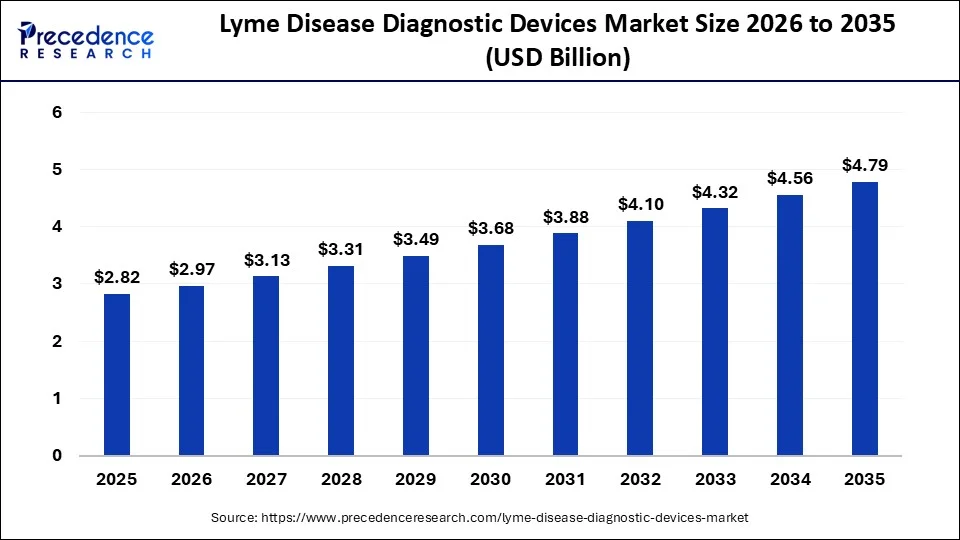
Lyme Disease Diagnostic Devices Market Key Takeaways
- North America led the global market with the largest market share of 40% in 2025.
- By Product, the serological test segment has held the largest revenue share in 2025.
- By Product, the lymphocytic transformation tests segment is anticipated to expand at a significant CAGR during the projected period.
- By End User, the hospitals segment has held the largest revenue share in 2025.
What is the Lyme Disease Diagnostic Devices Market?
Upsurge in incidence of Lyme disease, growing policy attention on improving health care services, and the advent of new diagnostic tests for Lyme disease pushes the market. Nonetheless, lack of knowledge of the disease and ineffectiveness in Lyme disease research reduces the market. Additionally, growth in key players' interests in Lyme disease diagnosis R&D is predicted to provide lucrative business growth opportunities. Moreover, increasing number of cases of tick-borne infectious diseases is boosting Lyme disease diagnostics market. Vector-borne diseases are illnesses caused by pathogens and parasites in human inhabitants. Each year greater than 1 billion individuals are infected and higher than 1 million people die from vector-borne illnesses. They can be infected by bacteria, parasites or viruses. More than 16% of the ailment and disability endured globally is because of vector-borne illnesses, with more than 50% of world's population presently assessed to be at risk of these ailments.
How is AI contributing to the Lyme Disease Diagnostic Devices Market?
Artificial Intelligence is not only able to diagnose Lyme disease better but also faster. The technology behind AI makes the whole thing more efficient. For example, with deep learning, even very subtle signs of the disease can be recognized while using predictive analytics in outbreak monitoring and public health planning. AI is able to support the development of personalized diagnostic practices, and in turn, clinical settings become more efficient, less prone to manual interpretation errors, and very innovative.
Lyme Disease Diagnostic Devices MarketGrowthFactors
- Growing prevalence of Lyme disease
- Increasing number of cases of tick-borne infectious diseases
- Growing investment in research and development
- Technological advancement
Lyme Disease Diagnostic Devices Market Outlook
Increasing awareness and rising disease burden are pushing the demand for advanced diagnostic devices that support improved clinical outcomes globally.
Sustainable development is a trend that emphasizes environmentally friendly analyzers and longer-lasting devices that promote responsible usage of diagnostic technology.
The adoption of improved testing has already begun in both the established and emerging markets, thus boosting international diagnostic capabilities.
Roche, Abbott, Bio-Rad, DiaSorin, QIAGEN, and Thermo Fisher Scientific are always pursuing new, groundbreaking diagnostic innovations and bringing them into the market worldwide.
The start-ups that are leading the way in the creation of new pathogen-detection techniques and the development of rapid point-of-care systems are, at the same time, forming collaborations with the established diagnostic companies that have a significant impact.
Market Scope
| Report Highlights | Details |
| Market Size in 2025 | USD 2.82 billion |
| Market Size in 2026 | USD 2.97 billion |
| Market Size by 2035 | USD 4.79 billion |
| Growth Rate from 2026 to 2035 | CAGR of 5.44% |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Product, End User, Region |
| Regions Covered | North America, Asia Pacific, Europe, Latin America, Middle East and Africa |
Lyme Disease Diagnostic Devices Market Segment Insights
Product Insights
Serological test segment recorded the prime market share in the global Lyme disease diagnostic devices market by type in 2025. Increasing use in the early diagnosis of Lyme disease diagnostics is the major reason for high market share of serological test. Other factors such as increasing investment in research and development is expected to increase the usage of serological tests over the estimate period.
The lymphocytic transformation tests are projected grow at the highest CAGR during the forecast period mainly due to new product launches in the near future.
End User Insights
The Lyme disease diagnosis is based on infection signs and symptoms, history of exposures to infected black-legged ticks, and laboratory tests. Serological tests can take several weeks to become conclusive after infection, indicating the need for new diagnostic tests that can identify the earliest signs of infection. Hospitals are well-equipped to handle patient flow and employ latest diagnostic and treatment techniques. These factors are primarily responsible for the greater market share of hospitals in the end-user segment of the Lyme Disease Diagnostic Devices Market. Public/private laboratories segment will expand at a significant CAGR during the forecast time-frame.
Lyme Disease Diagnostic Devices Market Regional Insights
The U.S. lyme disease diagnostic devices market size is estimated at USD 790 million in 2025 and is predicted to be worth around USD 1,380 million by 2035, at a CAGR of 5.74% from 2026 to 2035.
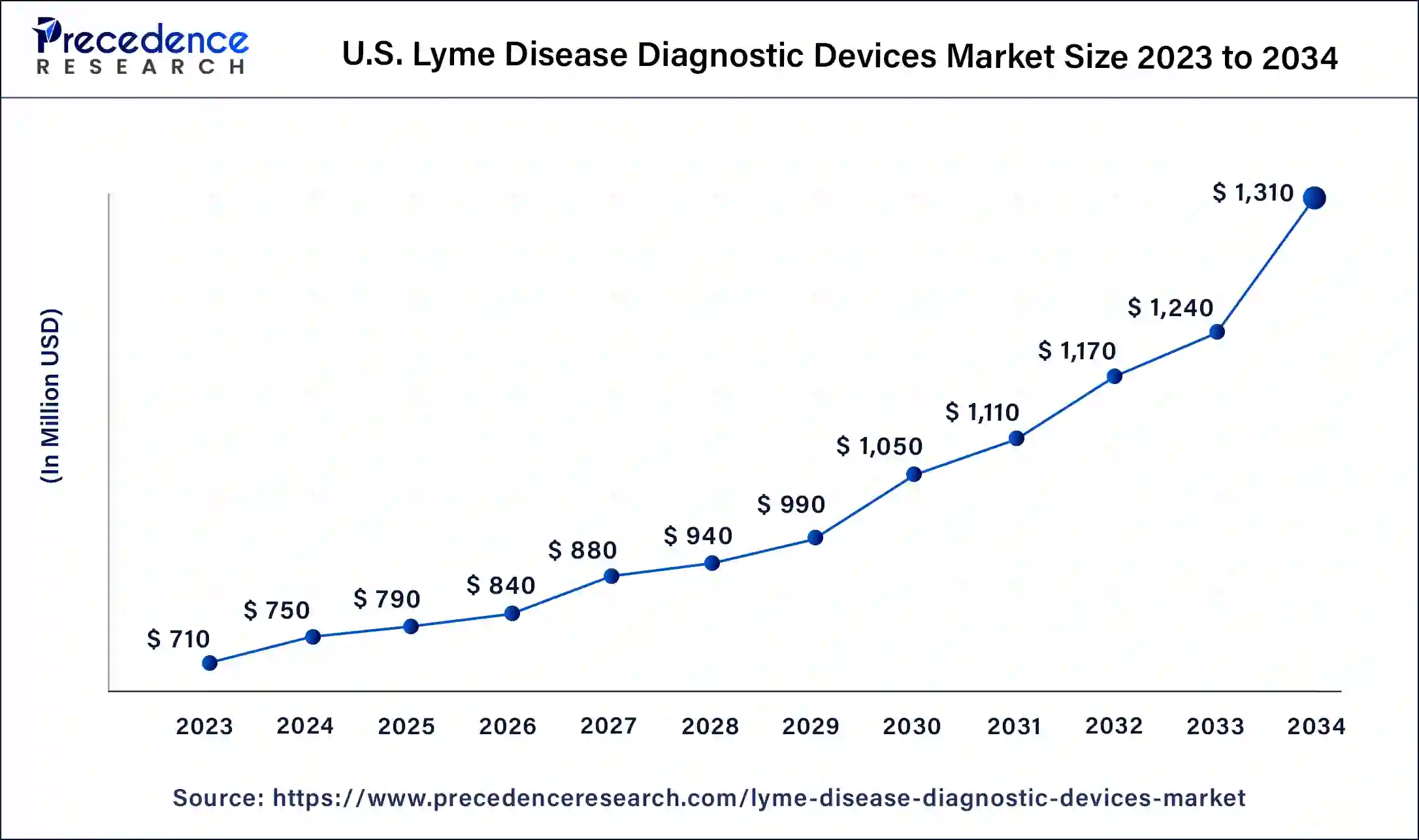
North America is Estimated to be the Largest Market for Lyme Disease Diagnostic Devices
The research report covers key trends and prospects of Lyme disease diagnostic devices products across different geographical regions including North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa. Geographically, Lyme disease diagnostic devices market is conquered by North America owing to high incidence of Lyme disease and existence of state-of-the-art healthcare infrastructure. On the other hand, Asia-Pacific is anticipated to witness a rapid growth rate, on account of increasing research and development investment by major market players and increasing awareness regarding tick-borne infectious disorders.
The North American market is driven by the presence of competent healthcare systems, strong diagnostic implementation, and an increasing awareness of the disease. The region's growth is supported by the continued development of new technologies and research to improve early detection and management.
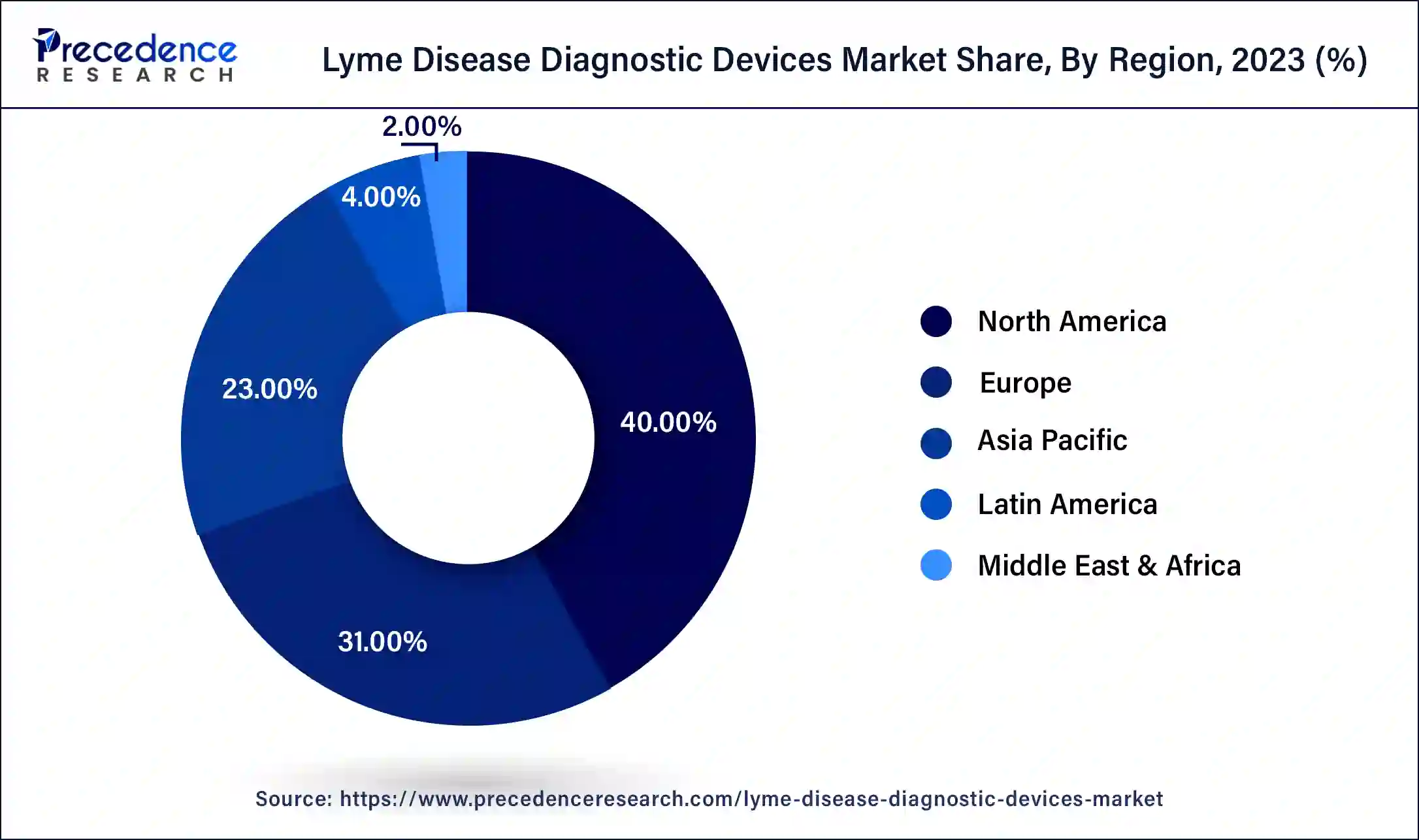
United States Lyme Disease Diagnostic Devices Market Trends
The United States has the advantage of government support that is strong, active research funding, and a considerable presence of diagnostic companies. Clear regulatory routes are a big plus for the innovation and research activities in the area of public health, which are also a big part of the reason for the latter innovations in the first place.
Europe is a huge market, and it is characterized by high disease prevalence, increased awareness, and healthcare investment. The advancements in diagnostics, the setting up of common testing rules, and the breakthroughs in research are all playing their part in developing the market in the region, and the early detection capabilities are even being enhanced to a greater extent now.
France Lyme Disease Diagnostic Devices Market Trends
France is on the route of increasing demand backed up by vigorous research activity, especially in the area of innovative preventive solutions. The collaboration between the research institutions is signaling a new development in diagnostics, which is in line with the current emphasis the country is putting on disease identification and management tools.
The APAC market is going through a rapid expansion phase, which is being driven by the growing awareness, the better healthcare infrastructures, and the increase in research participation. The measures taken to enhance the accessibility of diagnostics and the creation of inexpensive solutions are in play across both urban and rural areas, thereby facilitating the fast growth of the market in those regions.
India Lyme Disease Diagnostic Devices Market Trends
There is a noticeable increase in the potential of India as a result of the growing research and awareness in the high-risk areas. The possibilities are the wide-reaching nature of the low-cost diagnostics, the incorporation of better tests in the public health systems, and the tackling of the underreporting issue through enhanced surveillance and outreach education programs.
Lyme Disease Diagnostic Devices Market Value Chain Analysis
Discovering new methods and technologies and researching to develop diagnostics and their components.
Key Players: Abbott and Bio-Rad Laboratories
Conducting tests for safety and effectiveness, followed by applying for necessary regulatory approvals.
Key Players: Abbott, Bio-Rad, and Roche Diagnostics
Manufacturing diagnostic reagents and combining electronic parts to create working diagnostic devices.
Key Players: Thermo Fisher Scientific, DiaSorin S.p.A, and Meridian Bioscience
Making packaging materials that can protect the product and assigning serial numbers for traceability, compliance, and efficient inventory management.
Key Players: Quidel Corporation, Abbott Laboratories
Routing logistics and organized delivery of the approved devices to health facilities and ultimately to users.
Key Players: Quest Diagnostics and LabCorp
Lyme Disease Diagnostic Devices Market Key Players' Offering
Bio-Rad offers a full range of automated multiplex immunoassays for early and late-stage antibody detection through innovative system integration.
Thermo Fisher has an advanced product line of instruments, reagents, and software tailored to support a variety of diagnostic applications and lab settings.
Boulder Diagnostics’ SpiroFind test accurately identifies active Lyme disease by detecting the cellular immune response to the pathogen.
Other Major Key Players
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific, Inc.
- Boulder Diagnostics
- T2 Biosystems, Inc.
- Abbott
- Oxford Immunotec Global PLC
- Quidel Corporation
- Affymetrix Inc.
- Hoffmann-La Roche Ltd
- Graphene Frontiers
Recent Developments
- In October 2025, Twin brothers Jack and Will Goodreau launched Brothers Tick Kits, designed to safely remove ticks and protect families from tick-borne illnesses, inspired by their sister's Lyme disease fight. (Source: morningagclips.com)
- In March 2025, Antech launched truRapid™ FOUR, an in-house screening test for canine vector-borne diseases, detecting antibodies for Anaplasma, Ehrlichia, Lyme C6, and heartworm using whole blood, serum, or plasma. (Source:businesswire.com)
Market Segments Covered
By Product Type
- Serological Test
- Urine Antigen Tests
- Lymphocytic Transformation Test
- Immunofluorescent Staining
- Nucleic acid Test
By End User
- Hospitals
- Public/Private Laboratories
- Physician’s Office
By Geography
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- United Kingdom
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting