What is Medical Affairs Outsourcing Market Size?
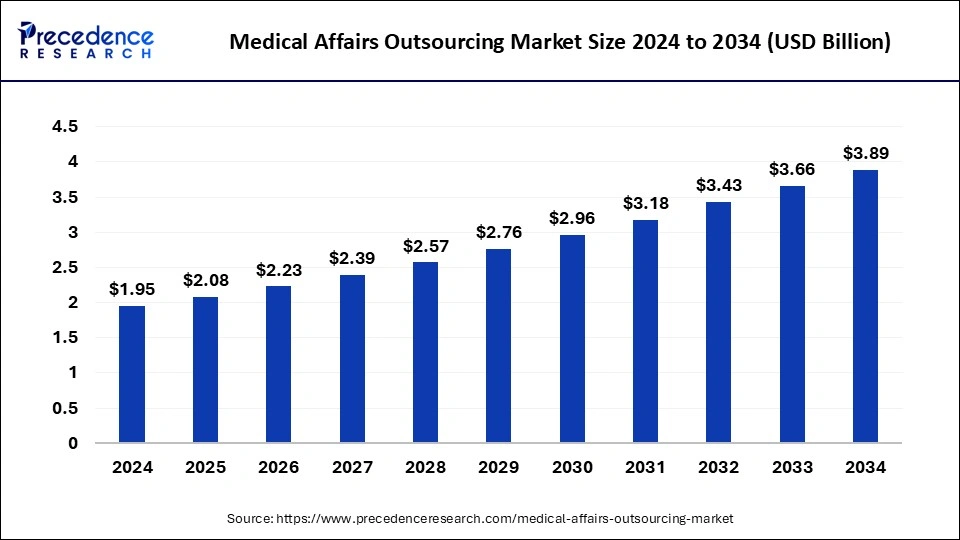
The global medical affairs outsourcing market size accounted for USD 2.08 billion in 2025 and is predicted to increase from USD 2.23 billion in 2026 to approximately USD 4.12 billion by 2035, expanding at a CAGR of 7.07% from 2026 to 2035.
Market Highlights
- North America region was valued at USD 710 million in 2024.
- By Industry, the pharmaceutical segment was valued at USD 1,029.61 million in 2024.
- By Services, the medical writing & publishing segment reached USD 654.62 million in 2024.
Medical Affairs Outsourcing Market Growth Factors
Current trends display an upsurge in subcontracting vital functional accomplishments predominantly in medical affairs including medical material, field-based medical teams and medical publications. Outsourcing medical affairs provides corporations cost-effective and flexible solution to address the budding issues and demands faced by the business. The regulatory setting is becoming rough due to amplified association among global regulatory bodies. As an outcome, compensations and drug approval need companies' medical affairs to gather snowballing amounts of complicated, real-life patient outcomes data to establish product value.
Several countries are progressively intensifying their R&D expenditure that has prompted expansion of innovative products in the market. With the cumulative stringency by the particular regulatory authorities globally, a rising partnership among clinical research organizations, the drug developers and contract manufacturing organizations has been perceived to alleviate risks. As CMOs and CROs assist the corporations into reduce risks, companies are able to emphasis on their fundamental dealings. Hence, escalating research and development activities plus patent expirations are considered to be major growth influencers driving the global medical affairs outsourcing market.
MarketScope
| Report Highlights | Details |
| Market Size in 2025 | USD 2.08 Billion |
| Market Size in 2026 | USD 2.23 Billion |
| Market Size by 2035 | USD 4.12 Billion |
| Growth Rate from 2026 to 2035 | CAGR of 7.07% |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Services, Industry, and Region |
| Regional Scope | North America, APAC, Europe, Latin America, MEAN, Rest of the World |
Segment Insights
Services Insights
Different medical services analyzed in this research report include medical monitoring, medical writing & publishing, medical information, medical science liaisons and others. Out of all these services, medical writing & publishing appeared as most promising segment and garnered maximum revenue share of 33.64% in 2025. Currently, plentiful of the big pharma corporations are subcontracting their clinical data management to specialized medical writing & publishing service suppliers. Demand for professional medical writers is projected to grow considerably due to escalating introduction of assorted pharma products in the market. As a result, augmented need for professional medical writers is projected as the crucial influencers for growth of medical writing & publishing market segment.
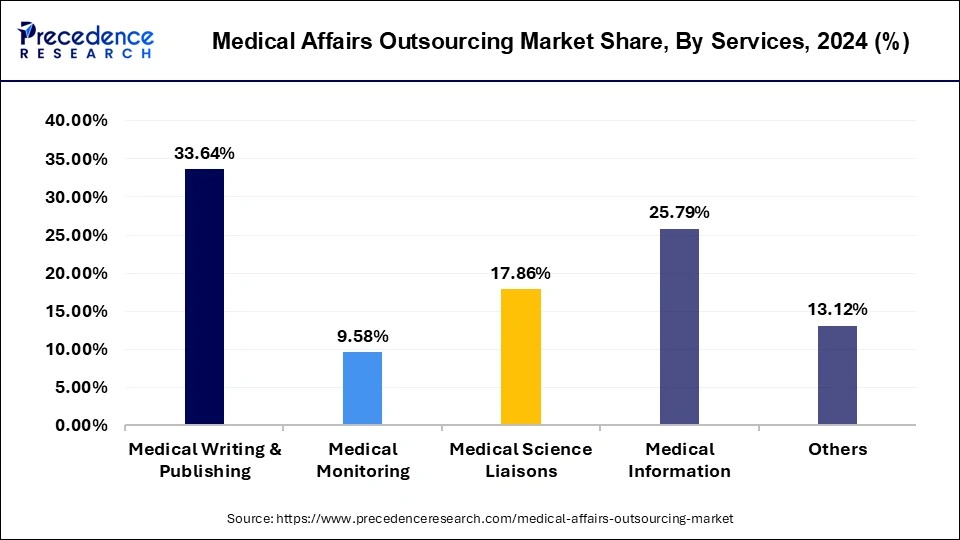
The Medical Science Liaisons (MSLs) services segment projected to record fastest developing growth rate during assessment period.These are medical specialists with cutting-edge scientific training and are experts in cooperating intricate medical data to a diversity of stakeholders. The biopharma business sector is concentrating on the core undertakings associated to drug development than non-core actions including medical affairs. In order to decreases pending and profit from professional medical affairs services, biopharmafrims are subcontracting these services to the CROs or contract research organizations. Biotechnology businesses are further continuing at a great pace and are anticipated to offer a resilient competition to mid-size pharma firms during next few years.
Global Medical Affairs Outsourcing Market Revenue, By Services, 2022-2024 (USD Million)
| By Services | 2022 | 2023 | 2024 |
| Medical Writing & Publishing | 564.37 | 607.61 | 654.62 |
| Medical Monitoring | 161.36 | 173.38 | 186.42 |
| Medical Science Liaisons | 329.72 | 338.41 | 347.58 |
| Medical Information | 443.24 | 471.52 | 501.94 |
| Others | 202.52 | 227.90 | 255.35 |
Industry Insights
Global medical affairs outsourcing market report assessed different end-use industries making most of these outsourcing services in this research report including biopharmaceutical, pharmaceutical, and medical devices among others.
Among these, pharmaceutical segment dominated the global market by accounting prominent share more than 52.86% of the total revenue generated by market in 2024. This growth is attributed to the growing demand of medical affairs service for the groundwork of drafts of fresh patents due to expiration of different existing patents.
Global Medical Affairs Outsourcing Market Revenue, By Industry, 2022-2024 (USD Million)
| By Industry | 2022 | 2023 | 2024 |
| Pharmaceutical | 898.33 | 961.40 | 1,029.61 |
| Biopharmaceutical | 228.76 | 245.56 | 263.76 |
| Medical Devices | 574.11 | 611.87 | 652.55 |
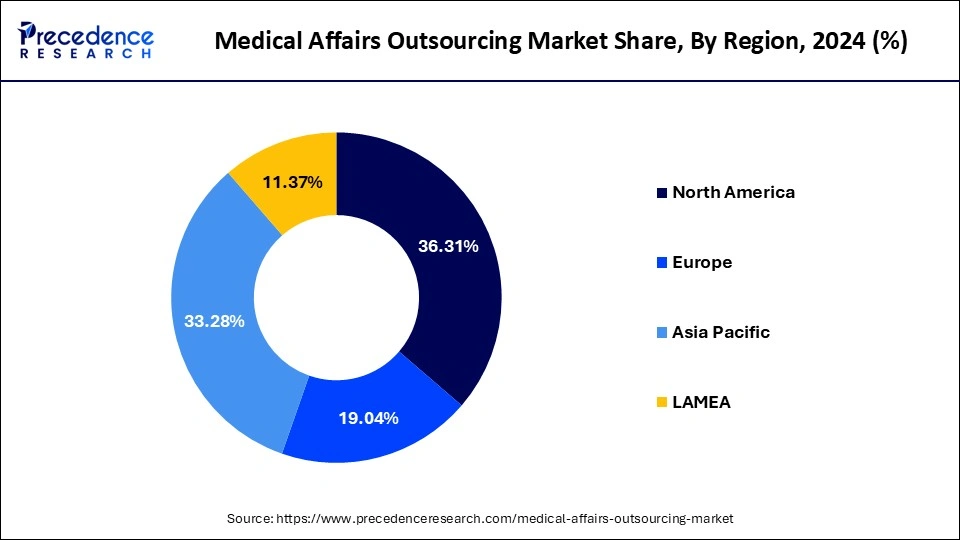
Regional Insights
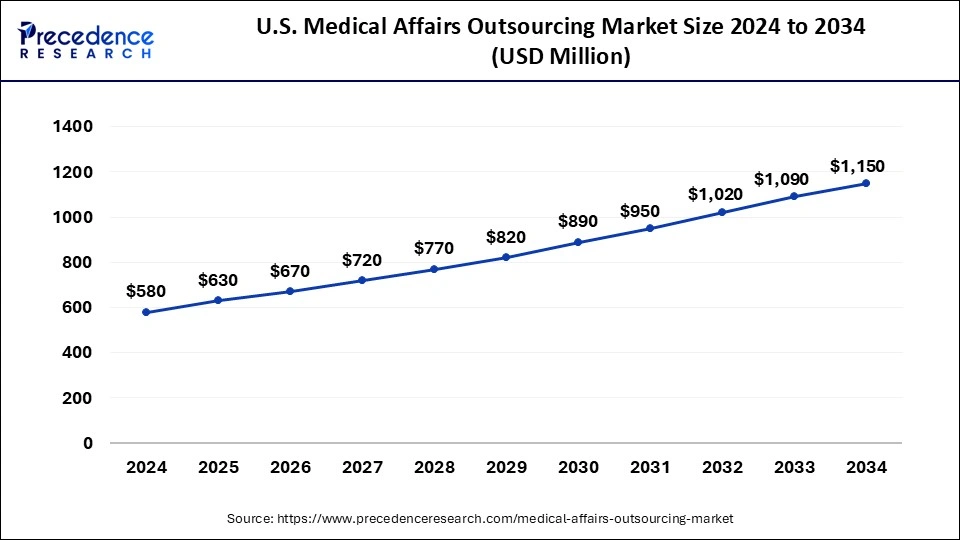
U.S. Medical Affairs Outsourcing Market Size and Growth 2026 to 2035
The U.S. medical affairs outsourcing market size is exhibited at USD 630 million in 2025 and is projected to be worth around USD 1217 million by 2035, growing at a CAGR of 6.81% from 2026 to 2035
North America Leads the Global Medical Affairs Outsourcing Market
North America region emerged as most dominating market place for the medical affairs outsourcing business in 2024 an estimated to mirror this predominance the period. Reasons such as vigorous FDA supervisors, existence of international life sciences andpharmaceutical giants and availability of talent pool are prospering the growth of the market in this region. The mounting figures of patent expirations along with escalating spending of research and development undertakings are some of the crucial factors persuading the event of the global medical affairs outsourcing market.
U.S. Medical Affairs Outsourcing Market Accelerates Amid Rising R&D Demands
The U.S. market is growing due to increasing clinical trial activity, rising regulatory complexity, and the need for cost-efficient expertise. Pharmaceutical and biotech companies are outsourcing medical writing, regulatory support, and post-marketing surveillance to focus on core R&D.
Strong FDA oversight, high R&D spending, frequent product launches, and the presence of skilled medical professionals are further accelerating market growth.
As rivalry in the international medical device market exaggerates, the U.S. medical businesses are progressively looking for means to decrease costs in manufacturing,clinical trials, research and development, and other medical services. Numerous Asian nationsbid a lower charge of labor such as China and India and skillful labor force. Merging this with the region's fast-growing markets and growing mature populations propose encouraging openings for the U.S. medical device corporations outsourcing their affairs to Asia Pacific. For instance, at present elderly population in Japan is around 20% against 12% aged population in the U.S.
- North America reached USD 706.61 million in 2024 and is anticipated to reach USD 1,342.31 million by 2034, registering a CAGR of 6.64% from 2025 to 2034.
- Asia Pacific was valued at USD 647.55 million in 2024 and is expected to reach USD 1,473.76 million by 2034, with a CAGR of 8.57% from 2025 to 2034.
- Europe was reached at USD 370.42 million in 2024 and is anticipated to reach USD 642.74 million by 2034, registering a CAGR of 5.68% from 2025 to 2034.
Asia-Pacific Emerges as a Cost-Effective Hub for Medical Affairs Outsourcing
The Asia-Pacific market is growing due to expanding pharmaceutical and biotechnology industries, rising clinical trial activity, and cost-effective service capabilities. Increasing regulatory harmonization, a large pool of skilled medical and scientific professionals, and growing adoption of outsourced medical writing, regulatory support, and post-marketing services are driving demand. Additionally, improving healthcare infrastructure and increased R&D investments by global companies are accelerating market growth across the region.
India Driving Growth in Medical Affairs Outsourcing with Skilled Workforce
The Indian market is expanding due to its large pool of skilled medical, regulatory, and scientific professionals, combined with cost-effective service delivery. Growing clinical trial activity, increasing pharmaceutical R&D investments, and rising demand for medical writing, pharmacovigilance, and regulatory support services are key drivers.
Favorable government initiatives, improving healthcare infrastructure, and strong adoption by global life sciences companies further support market growth.
Europe Expands Medical Affairs Outsourcing Market Amid Regulatory Complexity
The European market is expanding due to stringent regulatory requirements, increasing clinical research activity, and rising demand for specialized medical expertise. Pharmaceutical and biotechnology companies are outsourcing medical writing, regulatory affairs, and pharmacovigilance to manage complexity and reduce costs. Strong R&D investments, a well-established healthcare infrastructure, and the presence of leading life sciences companies are further driving market growth across the region.
The UK Leads in Specialized Medical Affairs Outsourcing Services
The UK market is expanding due to strong clinical research capabilities, a mature pharmaceutical ecosystem, and a highly skilled medical and regulatory workforce. Increasing regulatory complexity, post-marketing surveillance needs, and demand for cost-efficient medical writing and pharmacovigilance services are driving outsourcing.
Continued investment in life sciences innovation, supportive government policies, and strong collaboration between industry and research institutions further support market growth.
Medical Affairs Outsourcing Market Companies
- ICON plc
- Wuxi Clinical Development, Inc
- Pharmaceutical Product Development, LLC
- Ashfield Healthcare Communications
- Indegene Inc.
- IQVIA Holdings Inc
- The Medical Affairs Company (TMAC)
- SGS SA
- Syneos Health Inc
- ZEINCRO Group
Segments Covered in the Report
By Services
- Medical Monitoring
- Medical Writing & Publishing
- Medical Information
- Medical Science Liaisons
- Others
By Industry
- Medical Devices
- Pharmaceutical
- Biopharmaceutical
By Region
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Middle East & Africa
- Latin America
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content



