Multiple Sclerosis Drugs Market Size and Forecast 2025 to 2034
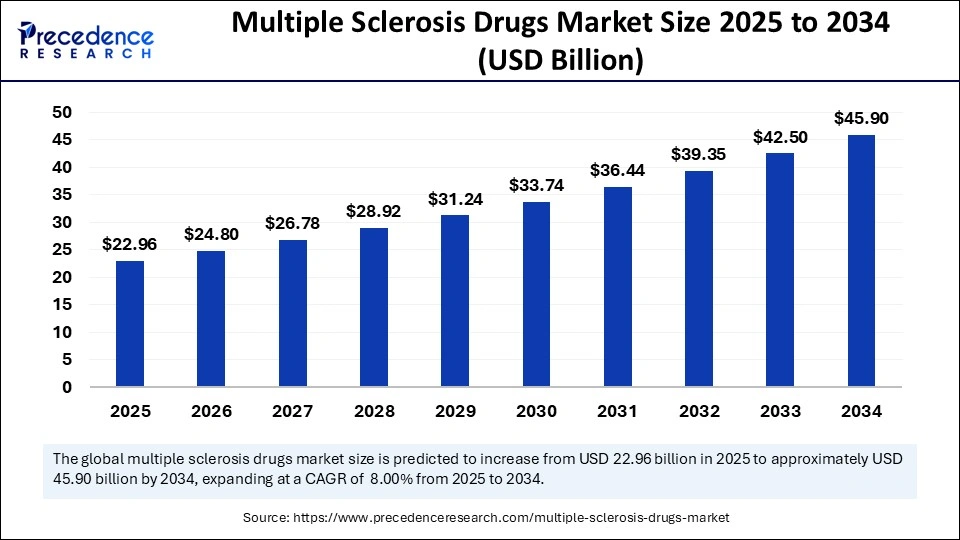
The global multiple sclerosis drugs market size accounted for USD 21.26 billion in 2024 and is predicted to increase from USD 22.96 billion in 2025 to approximately USD 45.90 billion by 2034, expanding at a CAGR of 8.00% from 2025 to 2034. The growth of the market is driven by ongoing clinical trials, increasing approvals from regulatory agencies, and rising investments in R&D activities for developing new therapies.
Multiple Sclerosis Drugs Market Key Takeaways
- In terms of revenue, the multiple sclerosis drugs market is valued at $22.96 billion in 2025.
- It is projected to reach $45.90 billion by 2034.
- The market is expected to grow at a CAGR of 8% from 2025 to 2034.
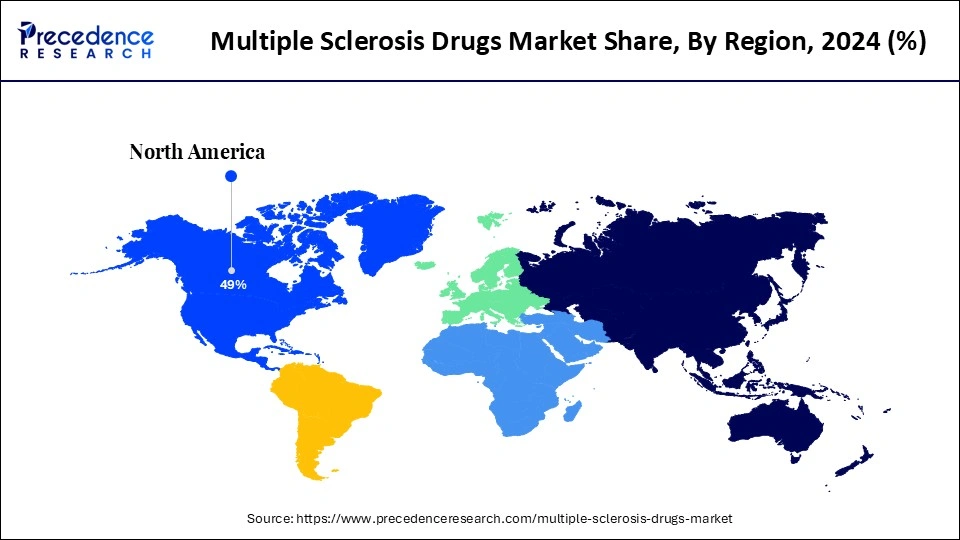
- North America dominated the global multiple sclerosis drugs market with the largest share of 49% in 2024.
- Asia Pacific is expected to grow at the highest CAGR between 2025 and 2034.
- By drug class, the immunomodulators segment held the biggest market share in 2024.
- By drug class, the immunosuppressants segment is expected to grow at the fastest rate during the forecast period.
- By route of administration, the injection segment accounted for the largest market share in 2024.
- By route of administration, the oral segment is anticipated to witness the fastest growth over the forecast period.
- By distribution channel, the hospital pharmacies segment dominated the market with the largest share in 2024.
- By distribution channel, the online pharmacies segment is anticipated to grow at the fastest rate during the projection period.
How does Artificial Intelligence Impact the Multiple Sclerosis Drugs Market?
Integration of Artificial Intelligence (AI) in multiple sclerosis (MS) drug development enhances efficacy and safety. AI-powered tools enhance drug development and treatment processes by enabling accurate diagnosis, personalized treatment strategies, and the development of new drug targets. AI algorithms can be applied in analyzing MRI images and image reconstruction to identify MS and detect subtle changes in brain tissue, indicating disease progression or response to treatment in patients. Identification of new blood or brain biomarkers can be achieved with the help of AI. Additionally, AI tools can accelerate drug discovery and development by identifying novel molecules, predicting the efficacy of drugs, and streamlining workflows in clinical trials.
- For instance, in April 2025, researchers from the University College of London (UCL) developed a new artificial intelligence (AI) tool, MindGlide, which can be applied to obtain valuable insights from brain images (MRI scans) for better interpretation and assessment of treatment progress in multiple sclerosis patients.
U.S. Multiple Sclerosis Drugs Market Size and Growth 2025 to 2034
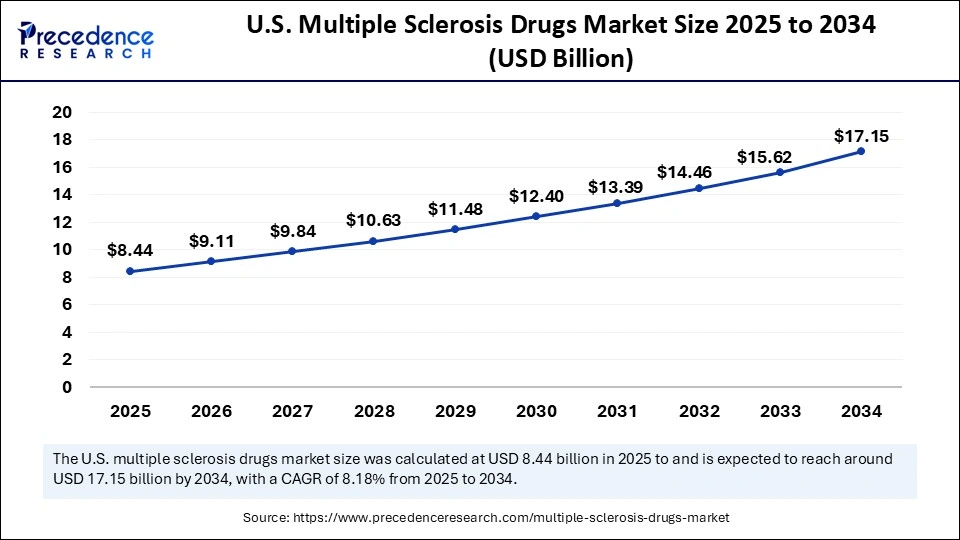
The U.S. multiple sclerosis drugs market size was exhibited at USD 7.81 billion in 2024 and is projected to be worth around USD 17.15 billion by 2034, growing at a CAGR of 8.18% from 2025 to 2034.
North America dominated the global multiple sclerosis drugs market by capturing the largest share in 2024. The region's dominance is mainly attributed to the increased prevalence of autoimmune conditions like multiple sclerosis. The presence of advanced healthcare infrastructure, continuous progress in developing innovative drugs and enhancing treatment strategies, huge investments by leading pharmaceutical companies, and supportive regulatory frameworks further bolstered the market.
The U.S. is a major contributor to the market. The multiple sclerosis drugs market in the country is flourishing due to factors such as the increasing number of cases of multiple sclerosis and increased access to advanced diagnostic technologies that support early detection. Increased healthcare spending, a strong emphasis on research and development, and the rising approvals for MS drugs further support market growth.
Asia Pacific is expected to witness the fastest growth over the forecast period. The growth of the market in the region is attributed to the increasing number of patients undergoing MS diagnosis, a well-established pharmaceutical industry, favorable government policies and initiatives enabling treatment access and reducing drug costs, and the expansion of distribution networks by implementing robust supply chain management strategies. In addition, rising investments by pharmaceutical companies in developing innovative treatments and rising government initiatives to advance healthcare infrastructure contribute to regional market growth.
Japan is expected to have a stronghold on the Asia Pacific multiple sclerosis drugs market. The rising prevalence of multiple sclerosis, especially neuromyelitis optica spectrum disorder (NMOSD), a specific subtype of MS, is a key factor driving the growth of the market in the country. There is a strong emphasis on developing targeted therapies, such as satralizumab and eculizumab. Moreover, the presence of a well-established healthcare system and the rising demand for personalized medicine are fueling the market's growth.
Market Overview
Multiple sclerosis (MS) can be referred to as a chronic autoimmune neurological disorder affecting the central nervous system (CNS), specifically the brain, spinal cord, and optic nerves. In MS, the body's immune system accidentally attacks the myelin sheath, which is the protective covering of nerve fibers, disrupting nerve signals, further leading to various neurological symptoms such as coordination problems and cognitive impairment among others. Ongoing advancements in research for improving patient survivability, increased success rates of clinical trials, demand for safe and effective treatments, growing awareness among the public about early diagnosis, advancements in diagnostic technologies, and the rising number of cases of multiple sclerosis are expected to propel the growth of the multiple sclerosis drugs market in the coming years.
Multiple Sclerosis Drugs Market Growth Factors
- Regulatory support: Regulatory agencies are supporting the development of new MS drugs. Access to MS drugs has been increased due to the growing approvals by regulatory bodies.
- Advancements in imaging technologies: Imaging techniques are widely applied in multiple sclerosis (MS), enabling early detection, monitoring of disease progression, and identification of potential therapeutic targets. Enhancements in Magnetic Resonance Imaging (MRI), such as advanced diffusion imaging (DWI/DTI), magnetization transfer imaging (MTI), myelin water imaging, glymphatic imaging, Magnetic Resonance Spectroscopy (MRS) as well as emerging techniques such as Optical Coherence Tomography (OCT), Coherent Anti-Stokes Raman Scattering (CARS) Microscopy and Positron Emission Tomography (PET) are offering comprehensive images of multiple sclerosis.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD45.90 Billion |
| Market Size in 2025 | USD 22.96 Billion |
| Market Size in 2024 | USD 21.26 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR 8.00% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Class, Route of Administration, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Drivers
Rising Incidence of Multiple Sclerosis
The rising incidence of MS across the globe is a key factor driving the growth of the multiple sclerosis drugs market. According to the report published by the World Health Organization (WHO) in August 2023, more than 1.8 million people have MS worldwide. This increase is attributed to various factors such as changing lifestyle patterns, lack of physical activity, reduced sunlight exposure, and genetic predisposition.
The rising awareness of MS among the public and healthcare professionals is increasing the number of patients undergoing MS diagnosis. Improved diagnosis methods for the identification of multiple sclerosis (MS) and advancements in treatments increased the survival rates of patients with MA. The growing demand for effective disease-modifying therapies (DMTs), investments by research organizations and governments fostering the development of innovative treatments, and ongoing clinical trials are further driving the market growth.
Restraint
Side Effects Associated with MS Drugs
While several multiple sclerosis drugs are considered effective in mitigating the associated symptoms, they may lead to severe side effects, which raises the questions about safety and efficacy of these medications for long-term use. Common side effects include flu-like symptoms, reactions at injection sites, and gastrointestinal issues. In some cases, these drugs can lead to serious events like cardiovascular problems, neurological complications, increased risk of infections like opportunistic infections, and rare conditions like Progressive Multifocal Leukoencephalopathy (PML). This raises concerns regarding the safety of these drugs, further restraining the growth of the multiple sclerosis drugs market.
- For instance, in January 2025, the U.S. FDA issued a Drug Safety Communication and also added a Boxed Warning regarding a rare but serious allergic reaction called anaphylaxis related to multiple sclerosis medication glatiramer acetate, which is branded as Teva's Copaxone and its generic alternative marketed as Glatopa.
Opportunity
Development and Commercialization of New Treatments
Manufacturers and researchers are focused on addressing the limitations of current treatments and therapies for multiple sclerosis by targeting specific subtypes like relapsing-remitting MS (RRMS), among others. Pharmaceutical companies are leveraging advanced technologies to improve the efficacy of drug formulations for enhancing patient compliance and safety. Furthermore, increased investments in advancing healthcare infrastructure and the rising development of affordable drugs and treatment options are creating lucrative opportunities in the market.
Drug Class Insights
The immunomodulators segment dominated the multiple sclerosis drugs market with the largest share in 2024. This is mainly due to the increased adoption of immunomodulators due to their high efficacy in managing MS symptoms. The increased prevalence of multiple sclerosis (MS), demand for efficient treatments, ongoing research activities for the development of innovative immunomodulatory drugs, and increased emphasis on personalized medicine further bolstered the growth of the segment. Advancements in immunomodulators and the development of new drug classes, such as sphingosine 1-phosphate (S1P) receptor modulators, which include Ozanimod and Siponimod, for the effective treatment of MS, drive segmental growth. Additionally, increased sales of drugs like Mavenclad and Tecfidera, as well as a surge in regulatory approvals for new immunomodulator drugs, are sustaining the segment's position in the market.
The immunosuppressants segment is expected to grow at the fastest rate during the forecast period. Enhanced comprehension of the immunological systems in the human body is facilitating the management of multiple sclerosis (MS), leading to the development of disease-modifying therapies (DMTs) for neuroprotection and targeting immune cells involved in MS, further offering better disease control. Regulatory approvals for innovative CD20-directed monoclonal antibodies like Ublituximab, as well as for Bruton's Tyrosine Kinase (BTK) inhibitors, are supporting segmental growth. Ongoing research activities aimed at developing effective immunosuppressant therapies, growing emphasis on combination therapies, and the development of humanized monoclonal antibodies further drive the growth of this segment.
- For instance, in March 2025, Sanofi Inc. declared the Breakthrough Therapy designation by the U.S. Food and Drug Administration for tolebrutinib, an experimental, oral BTK-inhibitor for the treatment of non-relapsing secondary progressive MS. The drug is also accepted for priority review by FDA and its decision will be made on or before 28 September 2025.
Route of Administration Insights
The injection segment held the largest share of the multiple sclerosis drugs market in 2024. Injectable drugs are widely used in management of multiple sclerosis, specifically in relapsing-remitting and secondary progressive forms, due to their enhanced capability for slowing disease progression, reducing the frequency of relapses, offering flexible dosing schedules, and enhancing long-term outcomes for patients. Injections allow for direct delivery of drugs to the bloodstream, reducing complications and enhancing patient outcomes. Moreover, a strong emphasis of researchers and manufacturers on developing safe and effective injectables, increased approvals for injectable drugs, and the heightened usage of infusion therapy bolstered the growth of this segment.
- For instance, in September 2024, Roche received approval from the U.S. Food and Drug Administration (FDA) for its OCREVUS ZUNOVO, a subcutaneous injection of the multiple sclerosis drug Ocrelizumab, for the treatment of both relapsing and primary progressive forms of multiple sclerosis. This new injectable version uses the ENHANZE drug delivery technology of Halozyme Therapeutics Inc. for the treatment of primary progressive multiple sclerosis (PPMS) and relapsing multiple sclerosis (RMS). The drug is administered twice-a-year in a 10-minute subcutaneous (SC) injection form.
The oral segment is anticipated to witness the fastest growth over the forecast period. The growth of this segment is attributed to the increasing patient preference for oral formulations. Oral formulations have lower side effects compared to injectable formulations. The surge in regulatory approvals for oral medications further supports segmental growth. Oral medicines are often more cost-effective and easier to administer than injectable medicines, making them the preferred choice. Furthermore, the rising investments in research and development activities for developing innovative oral disease-modifying therapies (DMTs), such as teriflunomide (Aubagio), fingolimod (Gilenya), and dimethyl fumarate (Tecfidera), contribute to segmental growth.
Distribution Channel Insights
The hospital pharmacies segment held the largest share of the multiple sclerosis drugs market in 2024. Hospital pharmacies offer various services, medication reconciliation, drug monitoring, and patient counseling, which are crucial for managing multiple sclerosis. These pharmacies often provide comprehensive information regarding medications, enhancing adherence to treatment, streamlining access to complex treatments, reducing healthcare costs, and providing support in shared decision-making. A multidisciplinary approach offered by hospital pharmacists by working with neurologists and other healthcare providers facilitates smooth communication, leading to enhanced care for MS patients. In addition, the easy availability of prescription as well as OTC medicines in these pharmacies attracts a large number of patients.
The online pharmacies segment is anticipated to grow at the fastest rate during the projection period. Online pharmacies provide a wide range of drugs from multiple brands. Online pharmacies offer a convenient way for patients to obtain their MS medications. These pharmacies provide faster doorstep delivery, attracting a large number of patients. Moreover, these pharmacies offer competitive pricing and discounts on MS medications. The growth of e-commerce and an increasing number of online platforms are improving access to MS medicines in remote areas. Online pharmacies provide consultation with doctors, reducing the need to visit hospitals.
Multiple Sclerosis Drugs Market Companies

- Bristol-Myers Squibb Company
- Teva Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Biogen
- Bayer AG
- F. Hoffmann-La Roche Ltd
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Novartis AG
- Takeda Pharmaceutical Company Limited.
- Horizon Therapeutics plc
- Sanofi
Latest Announcements by Industry Leaders
- In March 2025, researchers from Mass General Brigham announced the successful completion of a phase 1 clinical trial of its innovative nasal spray treatment called foralumab, which is a type of monoclonal antibody for the treatment of secondary progressive multiple sclerosis (SPMS). Dr. Tanuja Chitnis, director of Translational Neuroimmunology Research Center at Mass General Brigham, said, “Our research into this new nasal antibody treatment represents a significant step forward in managing MS symptoms. It has the potential to improve the lives of many patients by reducing inflammation and enhancing physical function.”
- In February 2025, Quantum BioPharma Ltd. announced the completion of the phase 1 multiple ascending dose clinical trial for its experimental drug Lucid-21-302, which is a first-in-class, neuroprotective, non-immunomodulatory compound for the treatment of multiple sclerosis (MS). The drug is a patented New Chemical Entity with a unique mechanism of action. Zeeshan Saeed, CEO of Quantum BioPharma, said, “We are excited about potential of Lucid-MS to protect myelin in MS patients as it represents a new direction in the treatment of this disease. By completing this trial and demonstrating safety in healthy participants, we are now closer to initiating a Phase 2 trial of Lucid-MS in people with MS. We are now looking ahead to our Phase 2 trial as we work towards our goals of drug approval and commercialization of Lucid-MS.”
Recent Development
- In March 2025, the National Health Service (NHS) England became the first healthcare system in Europe to introduce a new service of providing cladribine tablet for patients with active relapsing-remitting MS, after receiving approval from the National Institute for Health and Care Excellence (NICE).
Segments Covered in the Report
By Drug Class
- Immunomodulators
- Immunosuppressants
- Interferons
- Others
By Route of Administration
- Oral
- Injection
- Intramuscular
- Intravenous
- Subcutaneous
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting