FDA Approves Tapinarof Cream 1% (VTAMA) for Moderate to Severe Atopic Dermatitis in Patients Aged 2 and Older
The FDA has approved tapinarof cream 1% for the treatment of moderate-to-severe atopic dermatitis in patients aged 2 years and above. This drug, marketed by Organon as VTAMA, is a significant development in therapeutic offerings for this disease, especially in patients who had previously tried other treatments without success. VTAMA, an AHR agonist that is non-steroidal, binds and activates AHR receptors on skin and immune cells to address inflammation in atopic dermatitis and plaque psoriasis. No label warning, use duration restriction, or affected body surface area restriction is included in this treatment.
Tapinarof cream with a potency of 1% has received endorsement from the FDA to treat moderate-to-severe Alzheimer's disease (AD). The effectiveness of the cream was tested in two major clinical trials: ADORING 1 and ADORING 2. The main outcome of the clinical trials was a score of 0 or 1 on the Validated Investigator Global Assessment for AD (vIGA-AD) scale and at least a two-grade improvement. One of the secondary endpoints was the achievement of EASI-75 in terms of improvement of at least 75%. Results revealed significant efficacy demonstrated as follows: 45.4% of patients using tapinarof achieved the primary endpoint while 46.4% had clear or almost clear skin. The efficacy of tapinarof has proven to be consistent for all skin types, including those classified under the Fitzpatrick skin type system who can be termed as having darker skin tones. The secondary endpoint was also met because 55.8% of patients in ADORING 1 and 59.1% in ADORING 2 scored EASI-75, reflecting great improvement in symptoms. The ADORING 3 study is open-label, long-term, and included 728 patients for up to 48 weeks of continuous observations. Significant results yielded by ADORING 3 were complete disease clearance and treatment-free intervals. Safety data were also consistent with earlier 8-week trials.
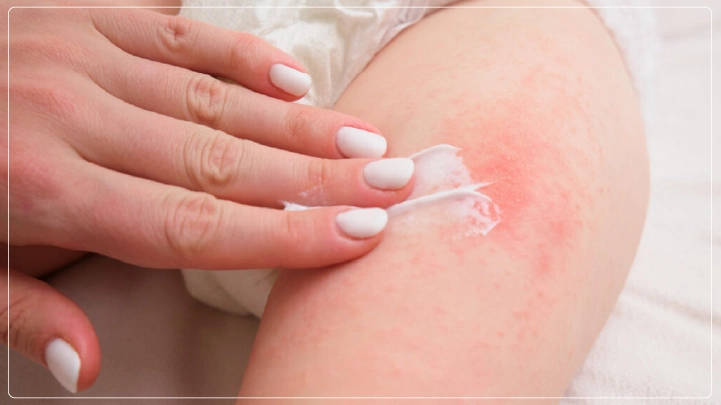
Much of the well-known ataxia symptom was reduced substantially by ADORING, thus showing the capacity of VTAMA to improve the quality of life for patients and their families. Tapinarof cream (1%) was well tolerated in all studies; the common side effects were upper respiratory tract infection (12%), folliculitis (9%), and lower respiratory tract infection (5%). There were no major concerns for safety, nor were there any restrictions on use.
The FDA has authorized tapinarof 1% cream (VTAMA), a topical, non-steroidal treatment option for atopic dermatitis in adult patients and children two years and older. It is a safe, effective, unmet-need treatment for patients suffering from moderate to severe Alzheimer's disease (AD). VTAMA has no scope limitations or contraindications; hence, it stands as a frontrunner therapy for atopic dermatitis in the market. The company is continuously looking for long-term studies and explorations of global market expansion opportunities. This approval is a major boost to the dermatology product portfolio.