Clinical Trial Management System Market is Representing 12.60% Growth by 2032
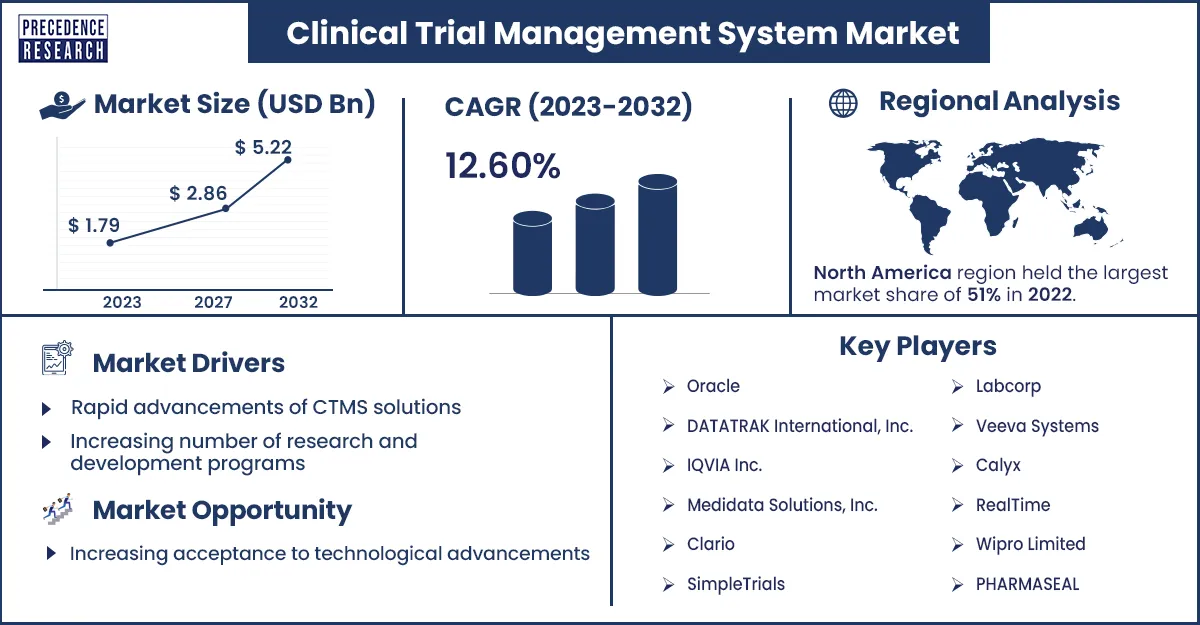
The global clinical trial management system market size was calculated at USD 1.79 billion in 2023 and is projected to hit around USD 5.22 billion by 2032, growing at a CAGR of 12.6% from 2023 to 2032. The increasing number of clinical trials and rising research and development activities may drive the clinical trial management system market growth.
Market Overview
The clinical trial management system market provides software for researchers to handle clinical trials efficiently. It helps report, track, and plan trial processes, such as regulatory compliance, data collection, and participant recruitment. The clinical trial management system facilitates drug development and regulatory standards, ensures trials adhere to protocols and enhances data accuracy. The increasing adoption of cloud-based clinical trial management system solutions and integration with electronic health records are anticipated to drive market growth. Electronic data capture systems, increasing focus on patient-centricity, and increasing demand for real-time data access are anticipated to boost market growth. In addition, modern technology adoption, effective medicine demand, and the increasing prevalence of chronic diseases are also expected to enhance the growth of the clinical trial management system market.
Improving data collection and accuracy drives market growth
Ensuring accuracy and data quality is of major importance for clinical trials. Incomplete and inaccurate data can compromise the reliability and validity of clinical trials. The clinical trial management system is automated alerts to detect missing data and checks the incorporation of robust data validation. This accurate resolution can help in improving reliability and data accuracy. In addition, the clinical trial management system offers audit trails, ensuring data integrity and permitting comprehensive review of data changes. These are the major key factors that are expected to drive the growth of the clinical trial management system market.
However, high-cost implementation and maintenance can restrain market growth. Implementing a clinical trial management system suffers from ongoing maintenance spending and substantial initial costs showing a major obstacle for mid-sized and small organizations. This financial problem hinders their ability to utilize and optimize clinical trial management system solutions and significantly challenges their participation in trial management and clinical research. These are the factors that are restraining the growth of the market.
Clinical Trial Management System Market Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 1.79 Billion |
| Projected Forecast Revenue by 2032 | USD 5.22 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 12.6% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Clinical Trial Management System Market Top Companies
- PHARMASEAL
- Veeva Systems
- Wipro Limited
- Real Time
- Calyx
- Signant Biotech
- Veeva Systems
- Labcorp
- Simple Trials
- Clario
- Medidata Solutions, Inc.
- IQVIA Inc.
- DATATRAK International, Inc.
- Oracle
Recent Development by Veeva Systems
- In October 2022, to deliver more efficient and faster trials to sponsors, Veeva Systems launched a clinical trial management system that generated 40 contract research organizations. This research organization included four of the top six global CROs, such as Syneos Health, the PPD clinical research business of Thermo Fisher Scientific Inc., Parexel, and Labcorp Drug Development.
Recent Development by Signant Biotech
- In October 2023, the leader in evidence generation for advanced clinical trials, Signant Health, launched Signant Biotech. These were the services that met the innovative demands of small and mid-sized biopharmaceutical organizations, as well as modern clinical trial investigation approaches using the software.
Regional Insights
North America dominated the clinical trial management system market in 2023. The growing advancements in the healthcare sector, government initiatives, increasing prevalence of chronic diseases, increasing geriatric population, and increasing demand for advanced software that can guide doctors to maintain records are expected to drive the market growth and demand in North America. The U.S. and Canada are emerging countries and help to enhance the demand for the market in the North America region.
In the U.S., clinical trial management services are in high demand due to the overage population and the prevalence of health diseases like chronic diseases. There are so many companies in the U.S. that provide new clinical trial therapies to the market quickly and safely through start-up timelines, accurate feasibility, and efficient and skilled staff with a customer-focused and flexible approach. The U.S. market players offer a better range of clinical trial services with 40 years of experience. Also, they offer clinical monitoring and international project management with skilled expertise. Some of the leading market players offer regulatory IND/NDA submission experience and regulatory guidance in the U.S., along with processes for each country and expertise in drug import regulations in North America.
In Canada, there are many clinical trial management system companies and a leading clinical investigator network. They have the largest-performing primary care researchers across the nation. Canadian experienced researchers have successfully completed thousands of phase ii-IV trials and trained in good clinical trial practice across the various therapeutic areas.
The geographical sector of Europe has also emerged as a major consumer of clinical trial management systems due to the growing advancements in the healthcare sector with government initiatives. There are various advantages of clinical trial management systems in Europe. The new regulations are made to streamline and simplify the clinical trial management and approval process in the European Economic Area. They help to solve the challenges and limitations associated with the European Clinical Trial Directive. The transition to European CTR exhibits a significant move in the European clinical trial platform. In Europe, sponsors can contribute to the advancement of clinical research and navigate the transition successfully by preparing to embrace effective change. The European Clinical Trial Regulation developed a combined approach to clinical trial reporting, assessment, and applications. A central electronic hub for all clinical trial communication, submissions, and applications is the major feature of the European Clinical Trial Regulation. These are the major and significant effective factors that are anticipated to drive the growth of the clinical trial management system market in Europe.
Market Potentials and Growth Opportunities
Increasing demand for real-time data access
For swift decision-making, market players in clinical trials highly demand real-time access to data. Clinical trial management system providing analytics capabilities, reporting and live monitoring. This necessity for quick data accessibility moves towards the industry’s drive towards responsiveness and efficiency in informed decision pathways, ensuring timely interventions and managing trials. These are the major opportunities expected to drive the growth of the clinical trial management system market in the coming years.
Increasing focus on patient-centricity
In clinical trial research, the shift towards patient-centricity emphasized the integration of recruitment models and patient engagement within clinical trial management system platforms. This initiative aims to improve retention rates and participant experiences by considering the preferences and needs of patients. Such integration enhances collaboration and greater management between study participants and researchers and boosts the overall clinical trial management procedure. These are also the driving opportunities anticipated to enhance the growth of the clinical trial management system market in the coming future.
Clinical Trials Management System Market News
- In February 2024, the world’s largest express transportation company and a subsidiary of FedEx Corp, FedEx Express, launched ‘The FedEx Life Science Center’ in Mumbai. The aim behind the launch was to set a success in the clinical trial management platform and supply chain globally.
- In February 2024, to evaluate emerging cancer screening technologies, the National Institute of Health launched a clinical trials management network. By investigating how to identify cancers earlier, the Cancer Screening Research Network supported the Biden-Harris administration's Cancer Moonshot.
Market Segmentation
By Type
- Side
- Enterprise
By Delivery Mode
- On premise
- Web-based CTMS
- Cloud based
By Component
- Software
- Services
By End User
- Clinical Research Organization
- Pharmaceutical
- Health care providers
- Biopharmaceutical companies
Buy this Research Report@ https://www.precedenceresearch.com/checkout/2082
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308