Snake Antivenom Market Size and Forecast 2025 to 2034
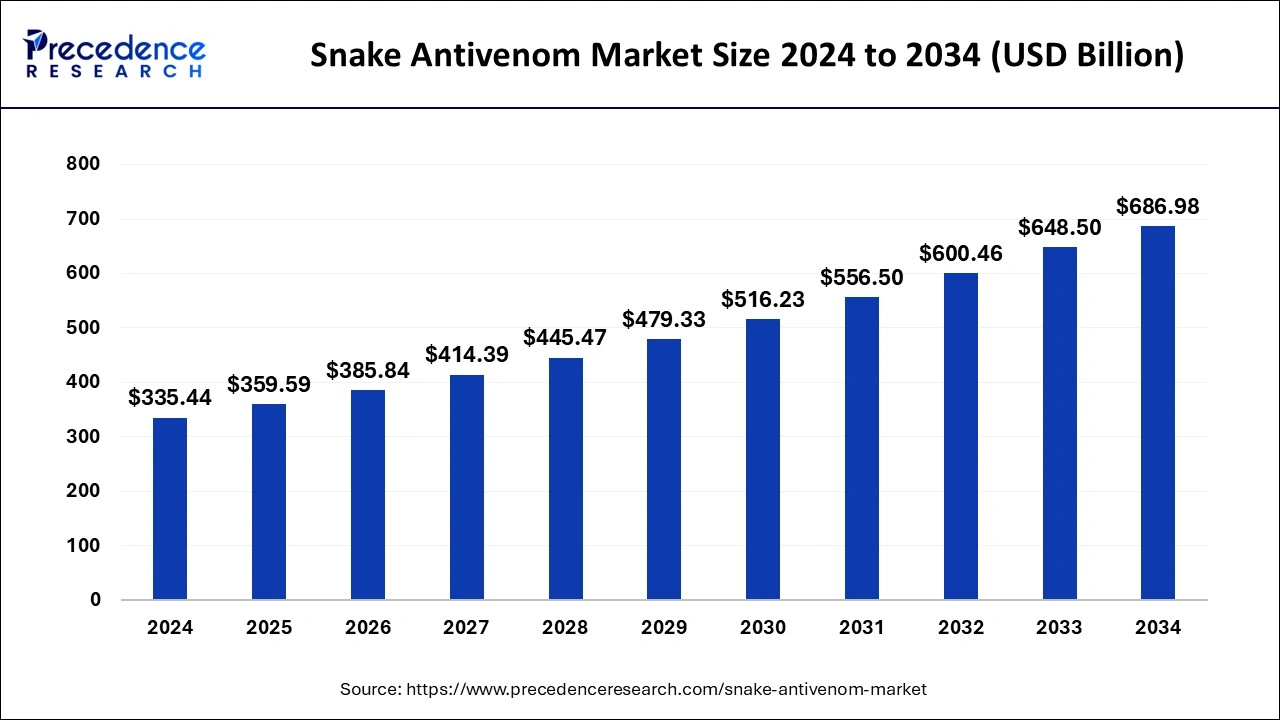
The global snake antivenom market size was estimated at USD 335.44 billion in 2024 and is projected to hit around USD 686.98 billion by 2034, growing at a CAGR of 7.43% from 2025 to 2034. The growth of the snake antivenom market is driven by the increasing demand for rapid-response antivenoms and the rising incidence of snake bites.
Snake Antivenom Market Key Takeaways
- The global snake antivenom market was valued at USD 335.44 billion in 2024.
- It is projected to reach USD 686.98 billion by 2034.
- The snake antivenom market is expected to grow at a CAGR of 7.43% from 2025 to 2034.
- North America contributed more than 42% of market share in 2024.
- Asia-Pacific is estimated to expand at the fastest CAGR between 2025 and 2034.
- By type, the polyvalent heterologous segment has held the largest market share of 59% in 2024.
- By type, the monovalent heterologous segment is anticipated to grow at a remarkable CAGR of 8.14% between 2025 and 2034.
- By application, the hospitals segment generated over 53% of market share in 2024.
- By application, the clinics segment is expected to expand at the fastest CAGR over the projected period.
Impact of Artificial Intelligence on the Snake Antivenom Market
AI plays a major role in the modulation of snake antivenom through research, manufacturing, and distribution. AI has been employed in the analysis of the elements present in venom to determine essential toxin parts more quickly and help find effective antivenom solutions. Its ability to analyze large amounts of data facilitates the research process, enhances antivenom efficacy, and shortens the development period. Antivenom manufacturing companies benefit from AI by automating production processes and monitoring the quality and quantity of antivenoms. Moreover, AI helps develop personalized antivenom treatments by analyzing individual patient profiles.
U.S. Snake Antivenom Market Size and Forecast 2025 to 2034
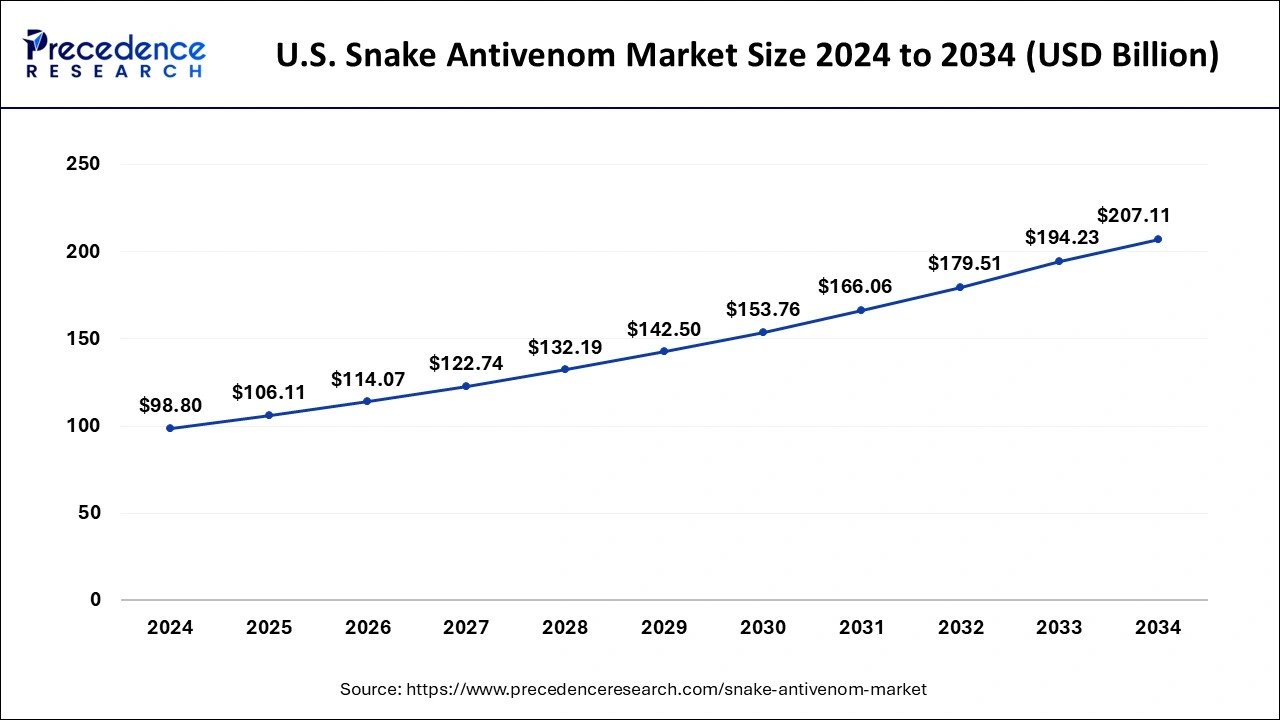
The U.S. snake antivenom market size was valued at USD 98.80 billion in 2024 and is expected to reach around USD 207.11 billion by 2034, poised to grow at a CAGR of 7.68% from 2025 to 2034.
North America holds a share of 42% in the snake antivenom market due to advanced healthcare infrastructure, robust research and development activities, and stringent regulatory standards. The region's well-established healthcare systems facilitate prompt diagnosis and treatment of snakebites. Additionally, a high awareness level among healthcare professionals and the general public ensures the demand for effective antivenom. Collaborations between research institutions and pharmaceutical companies further contribute to the development and availability of advanced antivenom formulations, solidifying North America's prominent position in the global market.
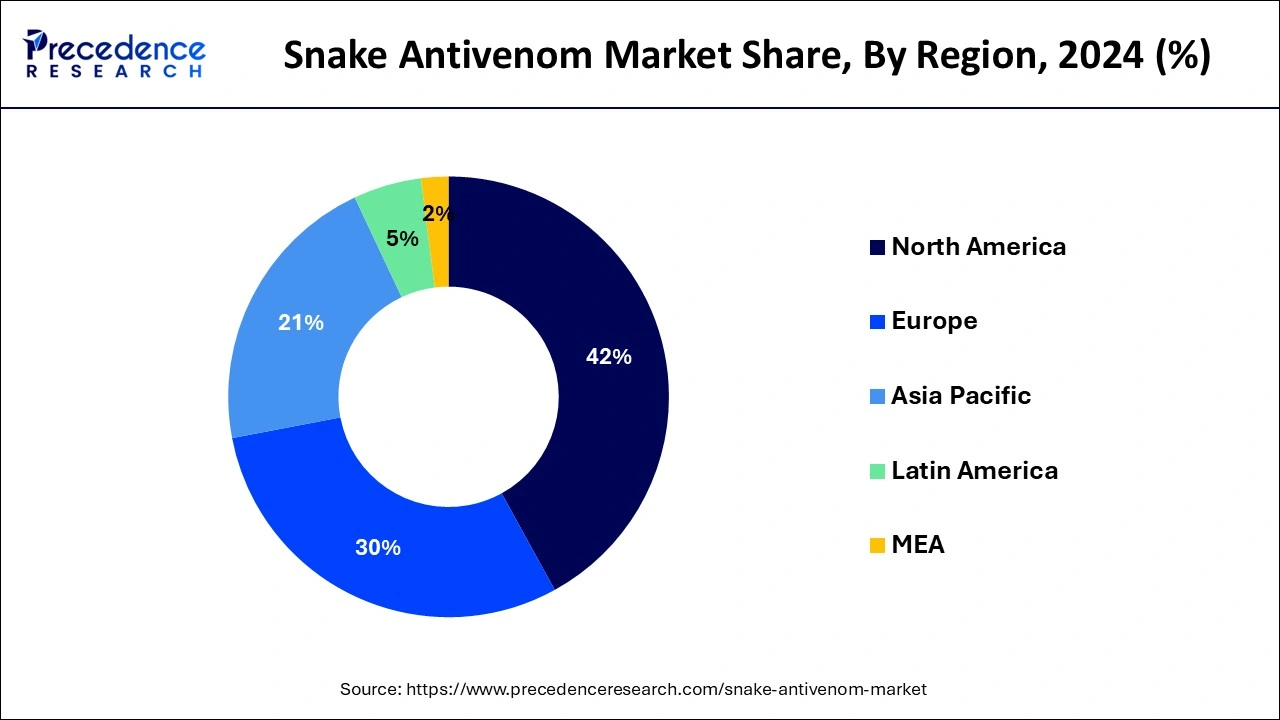
Asia-Pacific is projected to witness rapid growth in the snake antivenom market due to the high incidence of snakebites and the increasing awareness of the severity of envenomation. Rising healthcare initiatives, government support, and improved accessibility to antivenom contribute to market expansion. Additionally, the region's diverse snake species necessitate a variety of antivenom formulations, driving research and development. With these factors aligning, the Asia-Pacific market presents significant opportunities for addressing the pressing healthcare challenge of snake envenomation.
Meanwhile, Europe is witnessing notable growth in the snake antivenom market due to increased awareness and proactive healthcare measures. Despite a lower incidence of snakebites compared to other regions, rising awareness among the public and healthcare professionals about the potential risks of snake envenomation has led to a greater demand for antivenom. Moreover, advancements in research and development, coupled with improved regulatory frameworks, contribute to the market's expansion. The growing emphasis on preparedness and effective snakebite management positions Europe as a key player in the global snake antivenom market.
Market Overview
Snake antivenom is a life-saving medication designed to counteract the effects of snake venom after a snakebite. When a person is bitten by a venomous snake, the snake's toxins can rapidly spread through the body, causing various symptoms like swelling, pain, and even organ failure. Antivenom is created by injecting horses or other animals with small, harmless amounts of snake venom. The animals' immune systems produce antibodies against the venom, which are then harvested and purified to create antivenom. When administered to a snakebite victim, antivenom binds to and neutralizes the venom, preventing further damage and allowing the body to recover. Prompt administration of snake antivenom is crucial for a successful outcome in treating snakebites and minimizing the severity of symptoms.
Snake Antivenom Market Growth Factors
- The rising prevalence of snake envenomation cases worldwide is a significant factor driving the growth of the snake antivenom market. Regions with higher incidences of venomous snake bites contribute to an expanding market as the demand for effective treatments continues to grow.
- Ongoing research and development activities in the field of herpetology and biotechnology contribute to the discovery of new and more effective antivenom formulations. Technological advancements enhance the efficiency and safety of antivenom products, attracting both healthcare providers and patients.
- Government initiatives aimed at improving healthcare infrastructure, particularly in regions prone to snakebites, contribute to the growth of the antivenom market. Funding support for research, production, and distribution of antivenom helps ensure accessibility and affordability.
- Increasing awareness about the dangers of snakebites and the importance of timely and appropriate treatment fuels the demand for snake antivenom. Education programs, both for healthcare professionals and the general public, contribute to early detection and treatment.
- Collaborations between pharmaceutical companies, research institutions, and healthcare organizations play a pivotal role in advancing snake antivenom development and distribution. Partnerships facilitate knowledge exchange, resource sharing, and efficient distribution networks, positively impacting market growth.
- The globalization of antivenom supply chains ensures broader accessibility to effective treatments in various regions. Improved distribution networks and international collaborations enable swift responses to snakebite emergencies, fostering market growth on a global scale.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 7.43% |
| Market Size in 2025 | USD 359.59 Billion |
| Market Size by 2034 | USD 686.98 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Type and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Epidemiological trends
Epidemiological trends play a crucial role in driving the demand for snake antivenom in the market. With an estimated 2.7 million reported cases of snake envenoming globally each year, the increasing incidence of snakebites underscores the urgent need for effective treatments. As snakebite-related morbidity remains a significant health concern, particularly in regions where venomous snakes are prevalent, there is a growing demand for accessible and timely solutions.
The rise in snakebite cases prompts healthcare systems and communities to prioritize the availability and distribution of antivenom. Governments and health organizations allocate resources to address the epidemiological challenges, contributing to the market's growth. The demand surge reflects the critical role snake antivenom plays in mitigating the impact of snakebites, emphasizing the importance of continued research, development, and accessibility to meet the healthcare needs of affected populations.
Restraint
High cost of antivenom production
The high cost of antivenom production poses a significant restraint on the market demand for snake antivenom. The intricate process of manufacturing antivenom involves extracting antibodies from animals, such as horses, which requires specialized facilities and expertise. These factors contribute to elevated production costs, making the final product expensive. As a result, the affordability of snake antivenom becomes a challenge, particularly in regions where snakebites are more prevalent and healthcare resources are limited.
The financial burden associated with the production of antivenom can hinder its accessibility to a broader population, especially in lower-income areas where the need for effective snakebite treatments is the highest. This limitation may impede healthcare providers and affected individuals from obtaining an adequate supply of antivenom, leading to delayed or inadequate treatment. Efforts to address this restraint include exploring cost-effective manufacturing methods and advocating for financial support to make snake antivenom more accessible to those who need it most.
Opportunity
International collaboration
International collaboration is a key driver of opportunities in the snake antivenom market. By fostering partnerships between countries and organizations, the sharing of knowledge, resources, and expertise becomes more feasible. Collaborative efforts enhance the global response to snakebite emergencies, ensuring a coordinated approach to the production, distribution, and accessibility of antivenom. Through international collaboration, the pooling of research findings and technological advancements becomes possible, leading to more effective antivenom formulations. This not only benefits regions with established antivenom programs but also extends support to areas where snakebites are a significant public health concern.
Additionally, shared initiatives can address challenges related to regulatory standards and quality control, contributing to a more standardized and reliable supply of antivenom worldwide. Overall, international collaboration creates a platform for collective action, unlocking opportunities to improve the reach and impact of snake antivenom in diverse global contexts.
Type Insights
The polyvalent heterologous segment held the highest market share of 59% in 2024. Polyvalent heterologous snake antivenom is a type characterized by its ability to neutralize the venoms of multiple snake species. This segment addresses the challenge posed by the diversity of snake venoms. Trends indicate a growing preference for polyvalent formulations due to their broader coverage against various snakebites. Healthcare providers and manufacturers are increasingly recognizing the practicality of a polyvalent approach, streamlining treatment protocols and improving the efficiency of snake antivenom administration, contributing to the overall advancement of the snake antivenom market.
The monovalent heterologous segment is anticipated to witness rapid growth at a significant CAGR of 8.14% during the projected period. The monovalent heterologous segment in the snake antivenom market refers to antivenom formulations targeting a specific snake species, typically produced by using venom from a different species. This segment is characterized by its specificity, offering tailored treatments for individual snake bites. Trends in this segment involve ongoing research to develop more targeted and efficient monovalent antivenoms, ensuring better clinical outcomes. The focus on snake-specific formulations aligns with the goal of improving treatment precision and reducing adverse reactions, contributing to advancements in snakebite management.
Application Insights
The hospitals segment held a 53% market share in 2024. In the snake antivenom market, the hospitals segment refers to the use of antivenom treatments within hospital settings. Hospitals play a pivotal role in administering timely and critical care to snakebite victims. The trend in this segment involves increasing collaboration between healthcare facilities and antivenom manufacturers to ensure efficient distribution and availability. Additionally, ongoing efforts focus on enhancing hospital staff training for swift diagnosis and treatment of snakebite cases, further emphasizing the importance of the hospitals segment in addressing snake envenomation.
The clinics segment is anticipated to witness rapid growth over the projected period. In the snake antivenom market, the clinics segment refers to the administration of antivenom in clinical settings. Clinics play a crucial role in treating snakebite victims promptly and efficiently. A notable trend in this segment involves an increasing emphasis on establishing specialized snakebite treatment clinics. These clinics aim to provide targeted care, ensuring swift access to antivenom and comprehensive medical support. The trend reflects a growing recognition of the importance of dedicated clinical facilities in mitigating the impact of snake envenomation through timely and specialized interventions.
Snake Antivenom Market Companies

- CSL Limited
- Bharat Serums and Vaccines Limited (BSV)
- Rare Disease Therapeutics, Inc.
- Instituto Clodomiro Picado
- Vins Bioproducts Limited
- Inosan Biopharma
- Haffkine Bio-Pharmaceutical Corporation Limited
- MicroPharm Limited
- Instituto Butantan
- Serum Institute of India Pvt. Ltd.
- S. A. Altesa Lifesciences
- Flynn Pharma
- Instituto Nacional de Producción de Biológicos
- Vacsera
- Hetero Drugs Limited
Latest Announcement by Industry Leader
- In February 2024, Scripps Research scientists developed an innovative antibody that can neutralize the effects of lethal toxins found in the venoms of various snake species across Africa, Asia, and Australia. Senior author Joseph Jardine, PhD, assistant professor of immunology and microbiology at Scripps Research, said that this antibody targets one of the major toxins common to multiple snake species responsible for tens of thousands of deaths yearly. The development holds significant potential for addressing the high burden of snakebite-related fatalities and injuries, particularly in low- and middle-income countries, where the majority of such incidents occur.
Recent Developments
- In March 2024, Shri Apurva Chandra, the Union Health Secretary, launched the National Action Plan for the Prevention and Control of Snakebite Envenoming (NAP-SE) in India. Aiming to reduce snakebite deaths by half by 2030, the NAP-SE provides a comprehensive framework for states to develop action plans for managing, preventing, and controlling snakebites using the 'One Health' approach.
- In February 2024, scientists at the Indian Institute of Science (IISc) developed a synthetic human antibody capable of neutralizing a potent neurotoxin produced by the Elapidae family of highly venomous snakes, including the cobra, king cobra, krait, and black mamba. The team from IISc's Scripps Research Institute and the Evolutionary Venomics Lab (EVL) at the Centre for Ecological Sciences (CES) employed a technique previously used to screen for antibodies against HIV and COVID-19 to create this new venom-neutralizing antibody.
Segments Covered in the Report
By Type
- Polyvalent Heterologous
- Monovalent Heterologous
By Application
- Hospitals
- Clinics
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting