Systemic Inflammatory Response Syndrome Treatment Market Size and Forecast 2025 to 2034
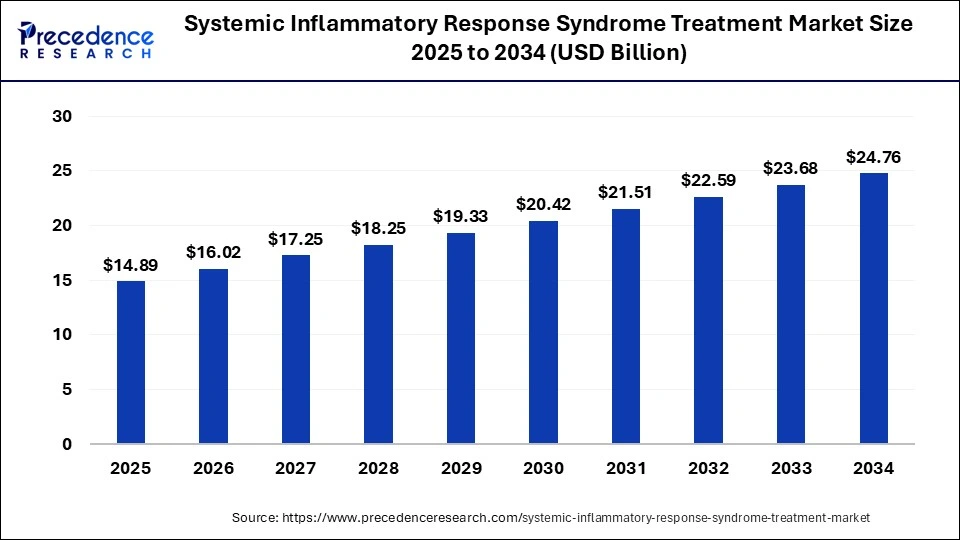
The global systemic inflammatory response syndrome treatment market size is valued at USD 13.85 billion in 2024 and it is projected to hit around USD 24.76 billion by 2034, poised to grow at a CAGR of 5.98% during the forecast period 2025 to 2034. The market growth is driven by an increase in the frequency of systemic inflammatory response syndrome and the prominence of systemic inflammatory response syndrome treatment to remain strong for urinary tract infection treatment.
Systemic Inflammatory Response Syndrome Treatment Market Key Takeaways
- The global systemic inflammatory response syndrome treatment market was valued at USD 13.85 billion in 2024.
- It is projected to reach USD 24.76 billion by 2034.
- The market is expected to grow at a CAGR of 5.98% from 2025 to 2034.
- North America has captured highest revenue share 38.89% in 2024.
- By appllication, the hospital & ambulatory surgical centers segment has held 62.15% revenue share in 2024.
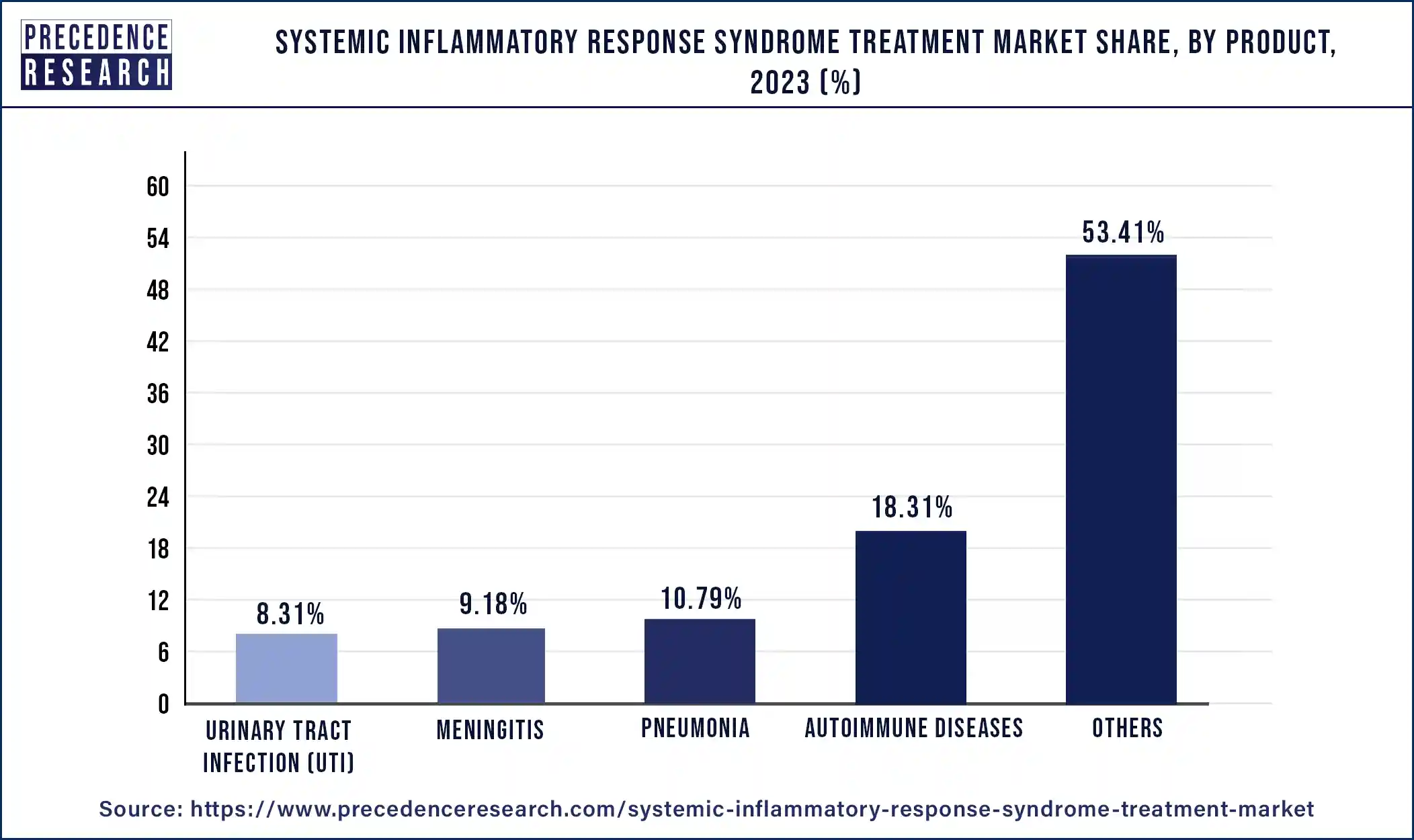
- By product, the autoimmune diseases segment has captured 18.31% revenue share in 2024.
- By product, others segment has garnered highest revenue share of around 53.41% in 2024.
Market Overview
The systemic inflammatory response syndrome treatment market is growing steadily and is driven by a rapidly increasing global prevalence of infections, trauma and critical illness which causes this syndrome. It can be described as a critical clinical response to a variety of insults whether they are infections, burns or pancreatitis that ultimately lead to severe inflammation. If left unchecked, this syndrome can progress to sepsis or multi-organ failure.
Overall, there is still a continued shift in focus toward early recognition and intervention with early diagnosis enabled by ongoing, advancing technology and increasing awareness and education of healthcare providers. The systemic inflammatory response syndrome treatment can vary from anti-inflammatory medications, immunomodulatory therapies, corticosteroids and supportive care in the ICU.
The significantly growing elderly population is contributing to the risk of immune-related complications and overall driving demand in this massive market. Pharmaceutical advances, such as biologics and new drug delivery systems, are now improving patient outcomes.
Systemic Inflammatory Response Syndrome Treatment Market Insights
One of the key factors for assessing post-surgical complications and end-organ dysfunction is systemic inflammatory response syndrome. Syndrome progression is linked to lengthy hospital stays, high incidence of multiple organ dysfunctions, and elevated morbidity. In cardiac surgery, systemic inflammatory response syndrome is among the popular post-operative complications, leading to organ failure or even death. It is expected that the prevalence of systemic inflammatory response syndrome among pediatric patients will increase in the near future. Increasing efforts by the key players to bring effective therapies and drugs for systemic inflammatory response syndrome can create huge growth opportunities in the target industry. As dilemmas about the mass of sepsis increase in systemic inflammatory response syndrome, market players are working to develop effective drugs and therapeutics within the scope of innovation. CytoSorb, a specific extracorporeal cytokine adsorber, is arising as a successful treatment to decrease inflammation and regulate the failure of essential organs such as the lungs, kidneys, heart and brain, amongst many other therapies. Biomarkers for early detection, treatment, and disposal of the condition have also been introduced as demand becomes more prominent for the initial-stage diagnosis of sepsis.
U.S. Systemic Inflammatory Response Syndrome Treatment Size and Growth 2025 to 2034
The U.S. systemic inflammatory response syndrome treatment market size is measured at USD 4.50 billion in 2024 and it is projected to hit around USD 8.44 billion by 2034, poised to grow at a CAGR of 6.49% during the forecast period 2025 to 2034.
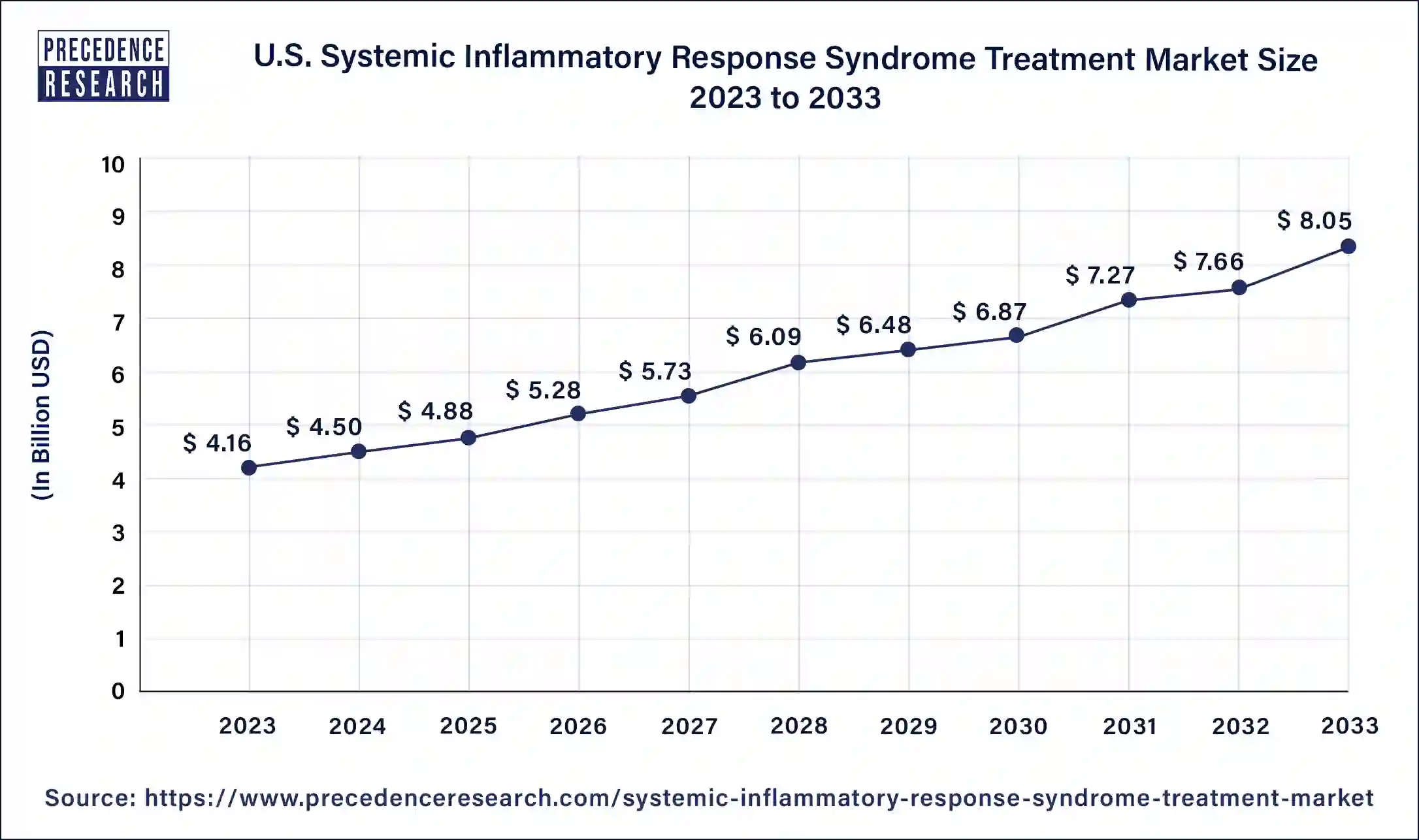
North America held the largest share of 38.89% in 2024 in the systemic inflammatory response syndrome treatment market. The market for Systemic Inflammatory Response Syndrome (SIRS) treatments in North America includes a broad spectrum of therapeutic therapies intended to control the intricate inflammatory response observed in critical illnesses, trauma, and sepsis, among other disorders. Antibiotics for the treatment of sepsis, corticosteroids, immunomodulators, and anti-inflammatory medicines are among the medications in this section that are intended to modulate the inflammatory response. This covers tools like respiration support ventilators, cardiovascular function tracking hemodynamic monitors, and severe organ failure management renal replacement treatment machines. Ongoing research & development initiatives to identify new therapeutic targets, enhance current treatments, and create cutting-edge medical devices define the SIRS therapy market in North America.
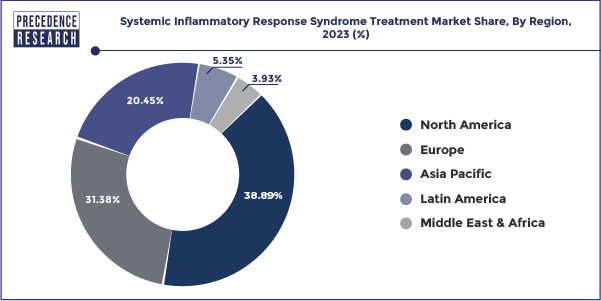
Asia pacific is expected to grow at the fastest growth rate during the forecast period. In the Asia Pacific area, the market for treating Systemic Inflammatory Response Syndrome (SIRS) is probably going to be dynamic and changing. Management of SIRS usually entails treating the underlying cause in addition to controlling the systemic inflammatory response. SIRS can result from a variety of underlying diseases, including infections, trauma, burns, or other inflammatory assaults. In SIRS, maintaining appropriate tissue perfusion and avoiding organ failure depend heavily on fluid management. This may entail the use of blood products, colloids, or crystalloids. This would be relevant to the market for intravenous fluids and blood products.
United States Systematic Inflammatory Response Syndrome Treatment Market Trends:
The United States is at the forefront of the global Systemic Inflammatory Response Syndrome (SIRS) treatment market, as a result of having a highly developed healthcare ecosystem, and incorporating advanced treatment protocols sooner than other nations. The high levels of incidences of sepsis, and critical care admissions, has increased the demand for rapid-response therapies that utilize advanced therapies such as corticosteroids, immunomodulators, and biologics.
Hospitals in the U.S. are potentially early adopters of innovative extracorporeal technologies, such as hemoperfusion systems, or ECMO devices, that improve patient response to severe SIRS cases, thus improving patient survival outcomes. Also, robust investment in research and development, active pharmaceutical leadership, and supportive regulatory framework like that of the FDA results in rapid clinical trail enhancements and product approvals. The U.S. will continue to be the benchmark for innovation and scalability in the SIRS treatment space.
China Systematic Inflammatory Response Syndrome Treatment Market Trends:
China is proving to be the fastest growing player in the SIRS treatment market and the government-led healthcare reforms, or the improvement of hospital readiness, and increased public awareness of the need to address inflammation early has stabilized and significantly improved treatment availability in China. Healthcare providers are taking notice with great enthusiasm, as evidenced by the growing clinical research and pharmaceutical collaborative activities, as they are increasingly consuming the advanced levels of therapies and diagnostics. The present growth of domestic biotech is also significantly advancing as new therapies cater to or are developed around local and regional demand.
Europe Systematic Inflammatory Response Syndrome Treatment Market Trends:
Europe is one of the leading strategic markets in SIRS treatments, although compressed into a fraction of market growth and market share behind North America. Germany, France and the UK are leading the European region, with strong clinical infrastructures and awareness of systemic inflammatory conditions and then experienced use of evidence-based treatment pathways. European system infrastructures also framed around reasonable reimbursement regimes for a more general access of both traditional and advanced SIRS therapies - corticosteroids, biologics, immunomodulators, and even extracorporeal support devices.
Systemic Inflammatory Response Syndrome Treatment Market Growth Factors
- Global growth in prevalence of inflammatory and infectious diseases: Growing burden of conditions such as sepsis, acute pancreatis and illness due to viral and bacterial infections are all collectively increasing the need for systemic inflammatory response syndrome treatment options.
- Improved diagnostic and monitoring technologies: Improved patient monitoring technologies in real time, inflammation biomarker recognition and early screening technologies help to improve the speed of recognition and subsequent of the systemic inflammatory response syndrome treatment.
- Isoflurane ICU and hospital bed-use growth: The complexity of the systemic condition that patients are being admitted for critical care has been increasing which means patients are in intensive care and being administered syndrome treatment protocols.
- Targeted Immunotherapeutics and biologics developments: Research and development continues in immunomodulatory medications, monoclonal antibodies and novel anti-inflammatory medications for more personalized and effective treatment paradigms.
- More clinical awareness and instruction: Education and awareness programs for clinicians and other health care professionals are supporting earlier detection and comprehensive management of systemic inflammatory response syndrome.
- Increased world-wide populations of geriatric patients: Older patients have less immune capacity to react to a systemic response which means that the therapies for this syndrome will be used for long term.
Supportive healthcare policies, with funding for R&D: Increased supportive funding for health research in critical illness contexts and supportive regulation continue to spur future market growth.
Market Scope
| Report Highlights | Details |
| Market Size in 2024 | USD 13.85 Billion |
| Market Size by 2034 | USD 24.76 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 5.98% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Application |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa (MEA) |
MarketDynamics
Driver
Personalized Medicine Approaches
Knowing a person's genetic predispositions can assist customize therapy to meet their unique requirements. Genetic testing can detect differences that impact the effectiveness, safety, and metabolism of medications. Personalized medicine relies heavily on biomarkers to help in disease diagnosis, prognosis, and treatment selection. Treatment choices in SIRS can be influenced by biomarkers such as levels of interleukin-6 (IL-6), procalcitonin (PCT), and C-reactive protein (CRP). Utilizing personalized immunotherapy techniques, SIRS is fought by the patient's immune system. Immunomodulatory drugs, like immune checkpoint inhibitors or monoclonal antibodies, can be customized according to the tumor's properties and the patient's immunological profile. Clinicians can minimize systemic side effects while achieving optimal therapeutic concentrations at the site of action by employing targeted drug conjugates or encapsulating pharmaceuticals within nanoparticles.
Restraint
Antibiotic Resistance
One major obstacle to treating systemic inflammatory response syndrome (SIRS) is antibiotic resistance. A dysregulated systemic inflammatory response to a variety of stimuli, including infection, trauma, or surgery, is the hallmark of SIRS, a complex illness. When bacterial infection is suspected or shown to be the underlying cause of SIRS, antibiotics are frequently utilized in its therapy.
Opportunity
Innovative Drug Delivery Systems
Novel drug delivery approaches are transforming the field of systemic inflammatory response syndrome (SIRS) treatment. SIRS carries a considerable morbidity and death rate and is defined by a dysregulated host response to infection or injury. It frequently culminates in multiple organ dysfunction syndrome (MODS). Vasopressors, supportive care, and broad-spectrum antibiotics have been the mainstays of traditional treatment strategies. Using medicine delivery systems based on nanotechnology is one creative strategy. Therapeutic medicines can be specifically delivered to regions of inflammation by using nanoparticles that have been designed to encapsulate them. Drugs are more effectively treated and have less systemic negative effects thanks to this tailored delivery. The creation of drug delivery systems with controlled release is another effective tactic. These systems provide the controlled and continuous release of therapeutic drugs, hence preserving therapeutic concentrations for a prolonged duration.
Indication (cause/trigger) Insights
The infectious causes play as the dominant segment of the systemic inflammatory response syndrome treatment market, largely due to the exceptionally high global burden of sepsis, pneumonia and bloodstream infections. These entities commonly lead to acute systemic inflammation, thereby accounting for most SIRS-related hospitalizations. The increased incidence of hospital-acquired infections and heightened rates of antibiotic resistance will contribute to immediate treatment requirements in this segment.
The non-infectious causes segment is quickly becoming the fastest growing segment as we see an increase in trauma, acute pancreatitis, burns and post-surgical complications. As the medical community continues to learn the triggers and develop better diagnostic processes for non-infectious cause for SIRS, treatment from this segment is becoming more in demand. This segment will continue to grow rapidly as critical care medicine advances in the years to come.
Mode of administration Insights
The injectable segment has the largest share of the systemic inflammatory response syndrome treatment market, due to its convenience and efficacy in emergency and critical care settings. There is much more ease of administration of intravenous corticosteroids, immunomodulators and biologics than there is with oral formulations-which is particularly important in the management of life-threatening inflammatory episodes associated with smooth systemic inflammatory response syndrome management.
The oral segment is the fastest growing segment, as it accommodates to the public demand of non-invasive and conversational post-acute care. The market for useful and effective oral anti-inflammatories and oral immunosuppressive formulations have advanced and come with a degree of anticipated future results to outpatient management in compliance with oral therapies. Long-term care is also more strategically supported in that the administration of drugs alters compliance in shorter stays and drugs like steroids are have moving available anti-inflammatories.
Product Insights
In 2024, the others segment held the largest share of 53.41% in the systemic inflammatory response syndrome treatment market. These treatments, which frequently include corticosteroids, NSAIDs (nonsteroidal anti-inflammatory drugs), and immunomodulatory compounds, aid in reducing inflammation in the body. vital for individuals experiencing severe respiratory distress, which is a frequent SIRS consequence. These tools assist medical professionals in keeping an eye on patients' cardiovascular health, which is essential for controlling shock and organ failure in SIRS. comprising plasma, platelets, and packed red blood cells to treat SIRS-related coagulation issues and anemia. such as procalcitonin (PCT) or C-reactive protein (CRP) testing, which can help diagnose and track the development of SIRS. including ultrasonography, CT scans, and X-rays to detect organ dysfunction and SIRS-related consequences.
Application Insights
The hospital & ambulatory surgical centers segment is the dominating segment of the systemic inflammatory response syndrome treatment market. They dominate with the largest percentage of the market share primarily due to advanced infrastructure, ICU table status, and medical specialists who can handle a severe systemic inflammation - from diagnosis to charges of complete care. This segment staying strong through with high ICU rates and difficult intra-operative/post-operative cases as they are the stronghold of SIRS management.
In contrast, the specialty clinics segment is the fastest-growing end-user segment. Specialty clinics break typical treatment models to provide the newest therapeutic strategies and treatments for inflammation control. The unique access to treatments and focus on inflammation solutions, non-emergent yet life-altering SIRS cases are becoming more desirable. As we see further investment in specialized care, disease specific clinical trials, and outpatient environments created solely for SIRS, specialty clinics are building more relationships and strengthening their growth trajectory.
Recent Developments
- In June 2024, CytoSorbents launched its PuriFi™ hemoperfusion pump in the European Union, following CE certification under the EU Medical Device Regulation. This innovative blood pump offers auto-priming, child- and adult-compatible tubing, and a touchscreen interface—designed for fast bedside setup in ICUs and cardiac suites to accelerate cytokine adsorption therapy. (Source- https://cardiovascularnews.com)
- In April 2024, results from the pivotal STAR?T trial were announced as a late-breaking presentation at the American Association for Thoracic Surgery meeting. The study a randomized, U.S.–Canadian trial evaluating the DrugSorb?ATR device for intraoperative removal of ticagrelor met its primary safety endpoint and showed reduced bleeding in elective CABG patients. (Source- https://ir.cytosorbents.com )
- During the second quarter in 2024, CytoSorbents secured its first E.U. orders 30 units of the PuriFi system—and obtained regulatory certification in Taiwan. The company also completed an international audit for Canadian market readiness and showcased STAR registry data at a major European conference.
(Source- https://ir.cytosorbents.com )
Systemic Inflammatory Response Syndrome Treatment Market Companies

- GlaxoSmithKline
- AstraZeneca
- CytoSorbents Corporation
- Cardinal Health
- Asahi Kasei
- ConvaTec
- CHIESI Farmaceutici
- Smith & Nephew
Segments Covered in the Report
By Indication (cause/trigger)
- Infectious causes
- Non-infectious causes
- UTI
By Mode of administration
- Injectable
- Oral
By End-user
- Hospital and ambulatory surgical centers
- Speciality Clinics
By Product Type
- Urinary Tract Infection (UTI)
- Autoimmune Diseases
- Meningitis
- Pneumonia
- Others
By Application
- Specialty Clinics
- Hospital & Ambulatory Surgical Centers
- Others
By Geography
- North America
-
- U.S.
- Canada
-
- Europe
-
- Germany
- France
- United Kingdom
- Rest of Europe
-
- Asia Pacific
-
- China
- Japan
- India
- Southeast Asia
- Rest of Asia Pacific
-
- Latin America
-
- Brazil
- Rest of Latin America
-
- Middle East & Africa (MEA)
-
- GCC
- North Africa
- South Africa
- Rest of Middle East & Africa
-
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting