What is the Clinical Trial Packaging Market Size?
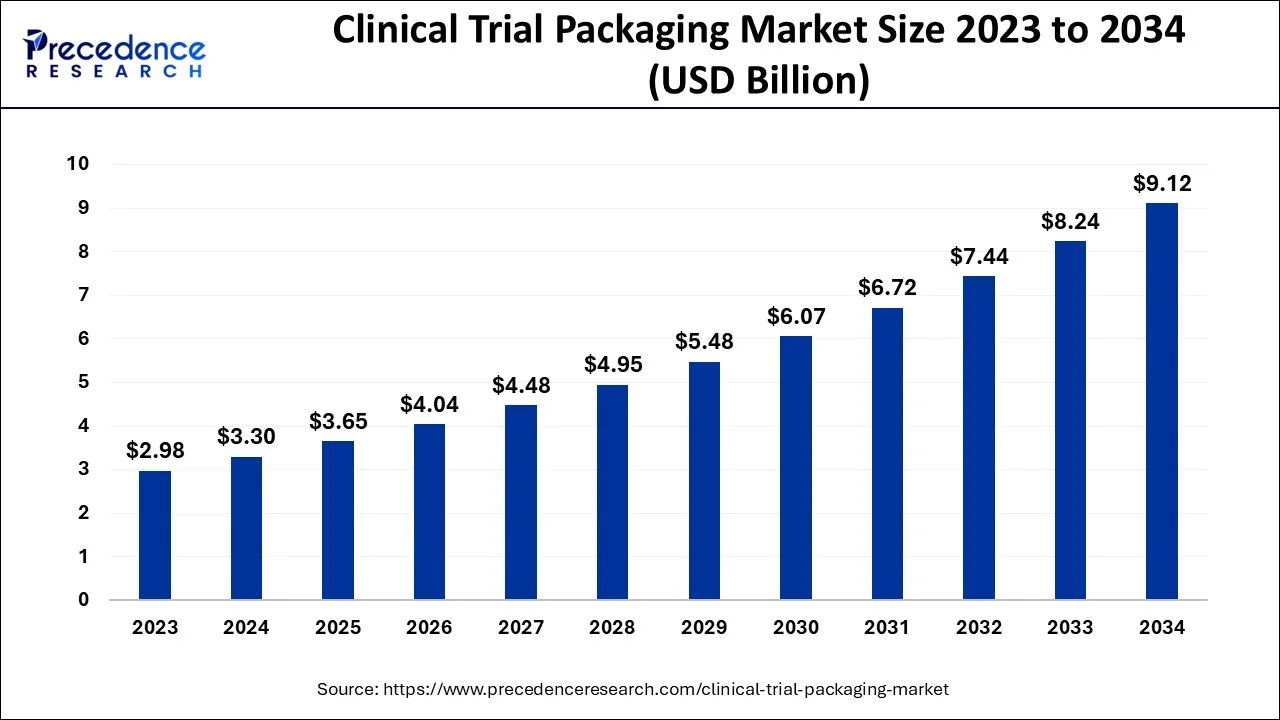
The global clinical trial packaging market size is calculated at USD 3.65 billion in 2025 and is predicted to increase from USD 4.04 billion in 2026 to approximately USD 9.12 billion by 2034, expanding at a CAGR of 10.70% from 2025 to 2034.
Clinical Trial Packaging Market Key Takeaways
- By packaging type, the bags & pouches segment held the highest revenue share of over 35% in 2024.
- By material, the plastic segment accounted for 37% revenue share in 2024.
- By end user, the research laboratories segment hit the highest revenue share of 44% in 2024.
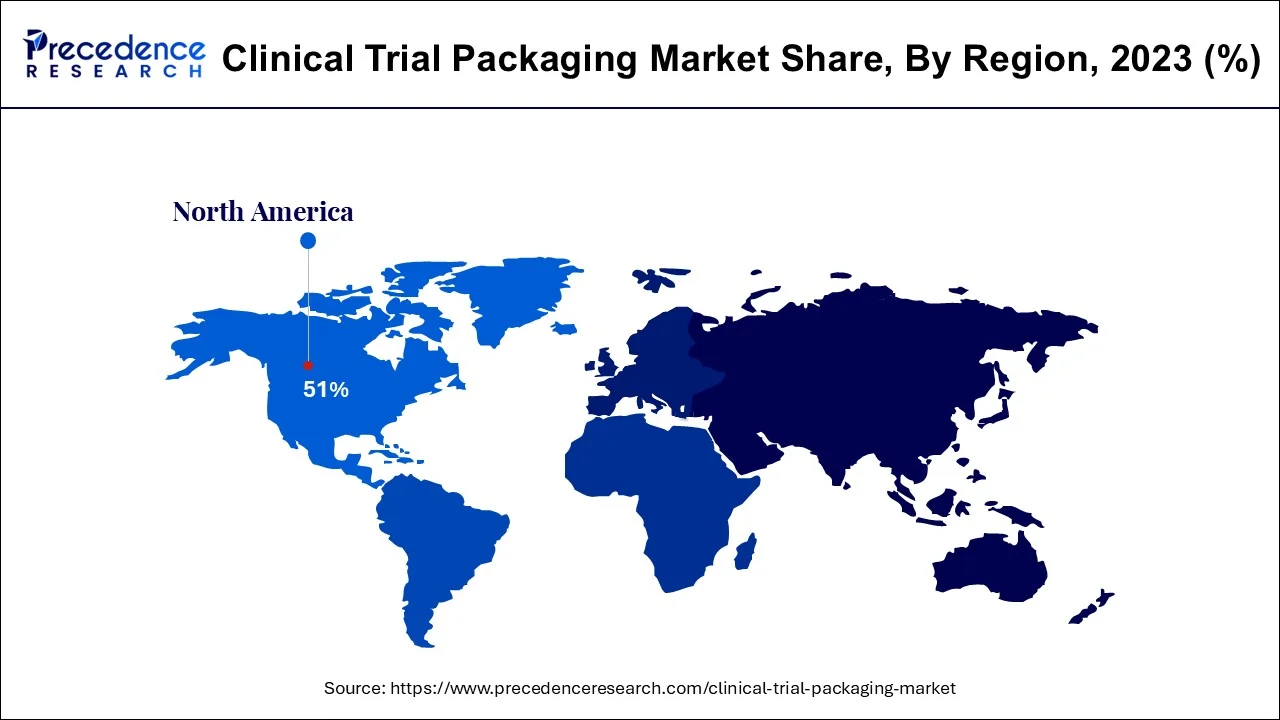
- North America region dominated the market with a revenue share of over 51% in 2024.
What Is Driving the Growth of the Global Clinical Trial Packaging Market?
Clinical trials are scientific examinations done to find better ways to screen for illnesses, diagnose them, and treat them. These clinical investigations may also show which therapeutic modalities are superior for specific illnesses or patient demographics. Clinical trials generate high-quality data that may be used to guide healthcare choices. The goal of clinical trials is to advance science. As a result, these studies follow stringent scientific standards that protect patients and assist in the creation of reliable clinical trial data. Since clinical packaging is used for research, functionality and utility are of highest importance. Rarely, such as in blind research when maintaining anonymity is crucial to the test's conclusion, visuals may even be completely ignored. Clinical batch packaging uses primary and secondary packing services. Pharmaceutical packaging products are becoming increasingly popular in international trade
The global market for clinical trial packaging has grown significantly over the past few years as a consequence of the rise in demand for clinical trial packaging. To help the industry establish a long-term solution and expand, the top producers of clinical trial packaging are investing in R&D. Through expanded distribution channels, mergers, and acquisitions, manufacturers in the clinical trial packaging industry are largely concentrating on extending their geographic reach. The growing importance of booklet labels is one of the changes in clinical trial packaging.
The COVID-19 epidemic had a favorable effect on clinical trial packaging since it raised demand for the packaging because to the enormous rise in vaccine development. Leading pharmaceutical firms conduct several clinical studies continuously to create and introduce new vaccines against the COVID-19 virus. Leading pharmaceutical firms have launched a large number of research trials to combat the pandemic as a result of the revolutionary COVID-19's widespread global distribution. As a result, there is a huge market for clinical trial packaging and other pharmaceutical packaging solutions.
- The primary drivers of the growth of the worldwide clinical trial packaging market are the internationalization of clinical trials and the harmonization of regulations, which has led to the outsourcing of clinical trials.
- Additionally, this business has expanded as a result of the rising demand for pharmaceutical services and goods. As a consequence of strict laws, their application, and the employment of suitable packaging methods to maintain pharmaceutical quality, the industry has grown.
- Due to patent expirations, the advent of new markets, and the biologics industry, the market is anticipated to expand.
- However, the sector's expansion is being constrained by the high expense of doing clinical trials and the absence of internationally coordinated trial registration systems.
Driven by a combination of increasing numbers of clinical trials and regulatory guidelines for drug safety and traceability, the global Clinical Trial Packaging Market is expanding rapidly. In conjunction with increasing R&D consistently occurring in biopharmaceuticals, rising demand for tamper-evident packaging, and a clear trend toward digitalizing the supply chain, the growth potential is promising. Moreover, the emergence of personalized medicine and biologics development has increased the global appetite for clinical trial packaging solutions, especially as they are positioned as advanced, compliant, and sustainable options.
Clinical Trial Packaging Market Growth Factors
Sustainability has been one of the most often debated subjects in the packaging value chain over the past ten years as a result of shifting regulatory requirements and a growing market demand for sustainable packaging solutions. Consumer behavior and purchasing decisions are still influenced by a variety of variables, particularly in the retail sector and, to some extent, the pharmaceutical business. Given that a number of market players are leaning toward the adoption of sustainable packaging materials, it is anticipated that a growing customer preference for sustainable packaging will continue to be one of the main factors likely to influence the development of the global for clinical trial packaging over the projected period.
Market Outlook:
What Lies Ahead in the Clinical Trial Packaging Industry?
The future of the clinical trial packaging industry appears bright. Given continued growth projected overall to 2032, driven by digital transformation initiatives, expanding biologics portfolio in drugs and devices, and personalized medicine considerations (pharma, CROs, and applicants) in trials, the clinical trial packaging market is anticipated to grow in the coming years. The advent and use of smart labels, sustainable packaging materials, and AI-based logistics bring new compliance and efficiency aspects to drug and device distribution in clinical trials.
- Industry Growth Projections: Due to the increasing number of clinical trials globally (including site expansions across the drug development continuum and trial duration) and consideration of standardized packaging/sustainability/traceability across the pharmaceutical supply chain, it is projected that the clinical trial packaging industry will continue to grow significantly.
- Sustainable Trends: Manufacturers are shifting to biodegradable, recyclable, and minimal-waste solutions to enhance and lower the environmental footprint of clinical trial management and supply chain processes. Initiatives that enhance the greening of labels (via recycling or reuse), terminally sterilizing shipments, or promoting carbon-neutral logistics are seen as compelling confidence-based competitive differentiators to the more traditional sustainability objectives of matter reduction, material composition testing in a clinical trial, shipping, or maintaining shelf life.
- Global Development: Pharmaceutical companies have expanded from North America and Europe into Asia-Pacific, Latin America, and Eastern Europe; thus, there will be a reliance on a package that addresses compliance, scale models, and logistics capabilities. Global connectivity is essential for packaging companies and CMOs or CROs to partner in a timely fashion and develop a sustainable process for global placements or for meeting a quality standard by an international contractor.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 3.65 Billion |
| Market Size in 2026 | USD 4.04 Billion |
| Market Size by 2034 | USD 9.12 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 10.70% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Clinical Trial Type, Packaging Type, Material, End User and Geography |
Market Dynamics
Key Market Drivers
- Development of New Drugs and Creative Ideas- The world market for clinical trial packaging is anticipated to expand as a result of recent adjustments made to the research and development procedures used by the pharmaceutical sector as well as the expanding need for cutting-edge goods and therapies. Additionally, the demand for glass bottles is rising as a result of the emerging problems associated with the usage of plastic. During the course of the expected period, it is predicted that increasing rates of infectious diseases such as cancer, HIV, and epilepsy will have a significant impact on the expansion of the worldwide clinical trial packaging market. It is anticipated that over the projection period, the growth of the global demand for clinical trials packaging would quicken due to a rise in R&D activities and government funding for the development of new pharmaceuticals.
- Clinical Trials Require Booklet Labels Frequently - Drug formulation innovation and R&D are the two main market drivers for clinical trial packaging globally. Many booklet labels and other packaging options are widely used in clinical research study packaging. The labeling options on these booklet labels may be utilized to represent a variety of information. Businesses that deal with packaging may drastically cut back on paperwork by using booklet labels. Additionally, booklet labels are utilized in clinical trial packaging to meet the labeling standards for supporting a variety of languages, global regulatory compliance, clear organization, and comprehensible text. It is projected that the need for these booklet labels in clinical trials would increase in the near future, boosting market development.
Key Market Challenges
- Absence of a centralized or standardized drug registration process: There is no centralized or standardized method for registering medications. Some of the issues that might limit the expansion of the clinical trial packaging sector include a lack of qualified labor and inadequate modern infrastructure in growing and developing nations. People are working harder than ever to develop clinical trial packaging that can withstand filling, shipping, handling, and other conditions.
Key Market Opportunities
- Increase in COVID-19: Clinical Trials for COVID-19 have increased A wide range of clinical trials are continuously conducted by top pharmaceutical firms to create and distribute new COVID-19 vaccines. Leading pharmaceutical firms have initiated a number of research projects to combat the epidemic as a result of the breakthrough COVID-19's widespread global distribution. As a result, there is a substantial market for clinical trial packaging and pharmaceutical packaging solutions. In developed markets in North America, Europe, and Asia, a rise in demand for clinical trial packaging items, including vials and ampoules, syringes, and bottles, among others, is also being seen. The COVID-19 pandemic has increased demand for clinical trials packaging services overall.
- Growing the market for pharmaceutical packaging products - The worldwide clinical trial packaging market has shown outstanding growth over the past 10 years as a result of increased global trade in pharmaceutical packaging goods. Clinical trial packaging has become more and more necessary in recent years. Clinical trial packaging producers are working on research and development projects that might produce industry-wide answers in the long run.
Clinical Trial Type Insights
The therapeutic and preventative category in the overall clinical trial materials and supplies market is predicted to develop at the quickest CAGR during the forecast period based on the kind of clinical research. A few of the drivers driving the market for clinical trial materials and supplies for therapeutic and preventative studies are the rising frequency of illnesses, the consolidation of biopharmaceutical businesses, and rising pharmaceutical companies' expenditure in R&D.
The markets for vaccines, medication development, therapeutic devices, biosimilars, therapeutic diagnostics, and therapeutic procedures are further divided into the therapeutic and preventive categories. Due to the rise in the prevalence of rare illnesses and the increasing burden of sickness on a population, the drug development segment is anticipated to expand at the quickest CAGR over this time.
Packaging Type Insights
The market was dominated by the bags & pouches segment in terms of revenue in 2024, while the others sector is predicted to grow at the highest CAGR during the forecast period. Over the last year, there has been a noticeable rise in the demand for blood bags, which are used to preserve blood and blood components. As the number of COVID-19 patients climbed, there was a two-fold rise in the need for convalescent plasma. In addition to COVID-19, other health-related problems are also driving up demand for intravenous (IV) and dialysis bags.
Material Insights
The plastic category led the clinical trial packaging market in 2024, while metal was expected to develop at the highest CAGR going forward. Polyvinyl Chloride (PVC), Polyethylene (PE), and other categories make up the type of plastic material. Additional types of polyethylene (PE) categories include high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP). The plastics sector was anticipated to account for more than half of the market by 2023.
Over the next seven years, the same market category is predicted to provide a total incremental potential of US$ 470 Mn with a 1.7x rise in value. Plastic packaging is being used more and more in clinical trials because it is convenient to utilize during transportation operations, affordable, and durable. Therefore, it is anticipated that the trend of using plastic packaging solutions would provide considerable commercial potential in the clinical trial packaging market throughout the projection period. Over the course of the projected period, it is anticipated that markets for PE sub-segments including HDPE & LDPE would grow rapidly.
Regional Insights
U.S. Clinical Trial Packaging Market Size and Growth 2025 to 2034
The U.S. clinical trial packaging market size is evaluated at USD 1.30 billion in 2025 and is predicted to be worth around USD 3.31 billion by 2034, rising at a CAGR of 10.91% from 2025 to 2034.
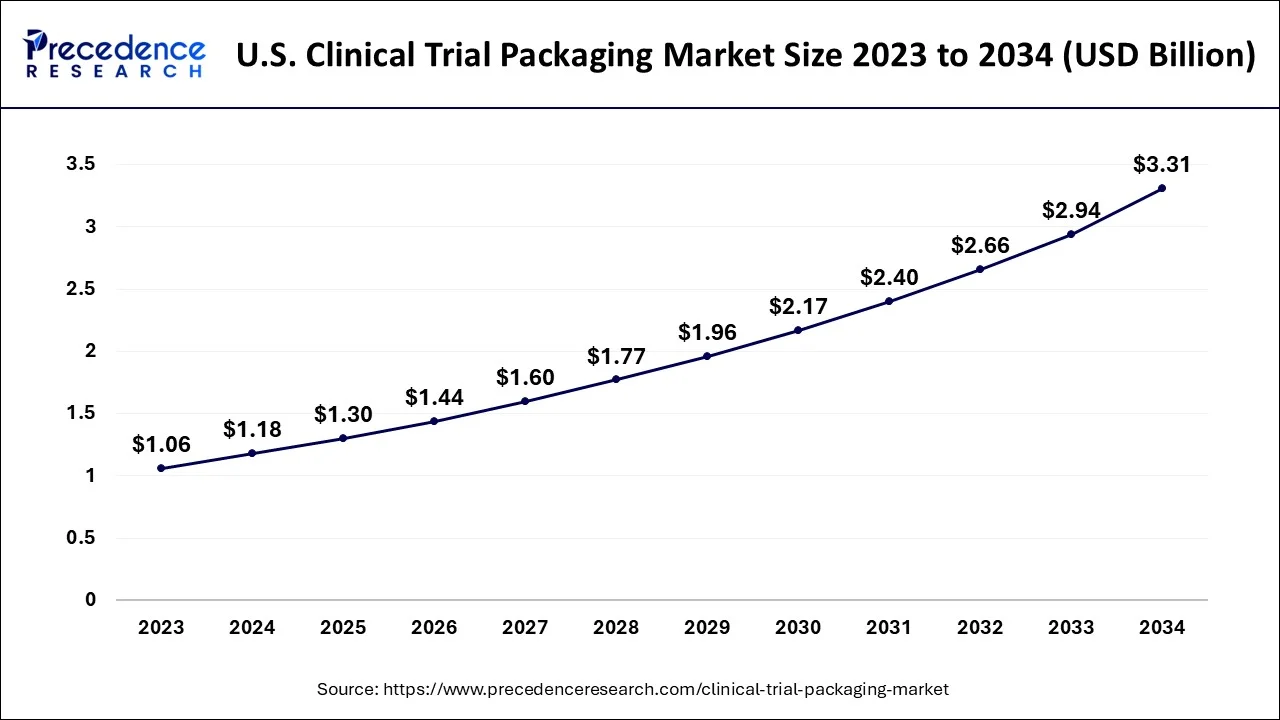
The primary forces driving the clinical trial packaging market's optimistic growth prospects are the region's well-established pharmaceutical industry and the extremely friendly regulatory environment for R&D activities. North America is anticipated to rule over the projected period with a market share of more than 51%.
North America: U.S. Clinical Trial Packaging Market Trends
The U.S. market is driven by a surge in clinical trial activity, especially for biologics, gene therapies, and personalized medicines. Smart packaging technologies, such as RFID, NFC tags, and temperature loggers, are increasingly used to ensure drug stability and traceability throughout the supply chain. There's also growing demand from contract packaging organizations (CPOs) and clinical research organizations (CROs), as pharmaceutical firms outsource more of their trial supply operations.
In the Asia Pacific area, there is likely to be considerable growth potential for clinical trial packaging. Leading pharmaceutical companies and rising economies with expanding production capacities are driving the APAC clinical trial packaging market.
Asia Pacific: China Clinical Trial Packaging Market Trends
China's clinical trial packaging market is expanding rapidly, fueled by a surge in R&D investments and a booming pharmaceutical industry. The globalization of clinical trials in China is increasing demand for packaging that meets international standards, including serialization, tamper-evident seals, and multi language labeling. Smart and temperature-controlled packaging is gaining traction, especially for biologics and vaccine trials, to ensure product integrity throughout the cold chain.
What Does the Clinical Trial Packaging Market Look Like in Latin America, and What is Driving Growth?
Latin America is steadily growing its clinical trial packaging market, driven by an increase in pharmaceutical research and international collaboration. Brazil, Mexico, and Argentina are emerging as key countries in hosting global clinical studies, as they have cost structures and an available patient base that make them attractive. Exciting government initiatives that improve R&D, along with more options in the healthcare continuum, are helping accelerate growth. Furthermore, local providers of packaging materials will increasingly adopt standards in order to compete for multinational sponsors.
Where are Clinical Trial Packaging Trends Emerging in Europe and why?
Europe retains a prominent share in the global clinical trial packaging market due to established pharma and biotechnology companies. The UK, Germany, and Switzerland are leading nations for clinical trials and have strong frameworks for managing drug development. Their legacies toward sustainable, serialized, and tamper-proof packaging are dictated by the current EU sustainability requirements.
They also have substantial capital and a determined focus on investing in and maintaining cold chain packaging concepts and traceability options that mitigate risk and sustain compliance to clinical study sites.
How Does the Clinical Trial Packaging Value Chain Improve Global Market Efficiency?
The clinical trial packaging of the product value chain can be characterized as a series of connected activities from the manufacturing of a clinical trial product to the management of the clinical trial packaging project and its distribution to clinical sites. It is imperative for pharmaceutical companies, packaging material suppliers, logistics suppliers, and regulatory bodies to efficiently coordinate the value chain process to secure the highest level of quality and integrity throughout the clinical trial.
- Proper Material Procurement and Package Development: Manufacturers actively pursue the procurement of proper materials considered to be sterile, high-quality, and temperature-resistant for clinical trial projects. Current and emerging design technologies are utilized to develop intelligent packaging that enhances serialization, labelling, and patient compliance.
- Regulatory Oversight and Quality Control: Regulatory authorities (e.g., the FDA, EMA, and WHO) have severe oversight over every package solution to make sure that they follow Good Manufacturing Practice (GMP) and ISO standards for labelling and storage.
- Temperature-Controlled Logistics: Some third-party logistics providers have the ability to provide cold and/ or temperature-controlled logistics. Such providers can guarantee controlled levels of specified temperature in environments like warehousing and during transportation to ensure drug efficacy. Real-time monitoring systems can augment supply chain transparency and accountability.
Clinical Trial Packaging Market Companies
- Bilcare
- Fisher Clinical Services
- WuXi AppTec
- PCI Pharma Services
- Almac Group
- PharMaterials
- PAREXEL
- Schreiner MediPharm
- Sharp Packaging
- The Coghlan Group
- Rubicon
- Westrock
- Xerimis
- Catalent
- Piramal Pharma Solutions
- Corden Pharma
- DMB Consultancy
- Körber Medipak Systems
- Sentry BioPharma
- NextPharma
- Mawdsleys
Recent Developments
- The New AmSkyTM blister system, Amcor's most recent invention, has the potential to increase the sustainable development of healthcare packaging. It was revealed in April 2021.
- To enhance and diversify its healthcare services, Ashfield, a unit of UDG Healthcare Plc, proposed the opening of three different business divisions in January 2021.
Segments covered in the Report
By Clinical Trial Type
- Therapeutic and Prevention
- Vaccines
- Drug Discovery and Development
- Therapeutic Devices
- Biosimilars
- Therapeutic Assays
- Therapeutic Procedures
- Diagnostics
- Diagnostic Assay
- Diagnostic Devices
By Packaging Type
- Syringes
- Vials & Ampoules
- Blisters
- Cold Forming
- Thermoforming
- Tubes
- Bottles
- Bags & Pouches
- Sachets
- Kits or Packs
- Others
By Material
- Plastic
- PVC
- PE
- HDPE
- LDPE
- PP
- Others
- Glass
- Metal
- Paper
- Corrugated Fiber
By End User
- Research Laboratories
- Clinical Research Organizations
- Drug Manufacturing Facilities
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting