Based DermaSensor Talks About Device for Skin Cancer Detection, Primary Detection to Get Easy
DermaSensor, the company behind the technology, published research confirming that its elastic scattering spectroscopy device can help primary care clinicians detect skin cancer more effectively. The study appeared in the Journal of the American Board of Family Medicine (JABFM) and investigated the accuracy and usefulness of the DermaSensor device among primary care clinicians in discerning malignant lesions from benign ones. The research confirmed that DermaSensor is capable of correctly identifying benign lesions, which assists in early detection of malignant cases, and ensures quick referral of patients who must see a specialist regardless of distance from their home.
In the study, three primary care clinicians situated in a rural area assessed skin lesions of concern in 155 patients and thus assessed 178 lesions using the DermaSensor compared to a biopsy or a teledermatologist panel. The device gave a sensitivity of 90.0% and a specificity of 60.7%, significantly surpassing the sensitivity of the clinicians’ Standard of Care Management which was 40.0% but with similar specificity to the clinicians who were 84.8%. When focused on pigmented lesions, the device specificity dropped to 76.9%, which was, however, in very high alignment with the teledermatologist panel, implying that the device can be reassuring for both patients and physicians when assessing lesions.
The mobile application is expected to get FDA approval by January 2024, and DermaSensor is a low-cost portable gadget employing spectroscopy and smart algorithms to check for cancer in skin lesions within minutes.
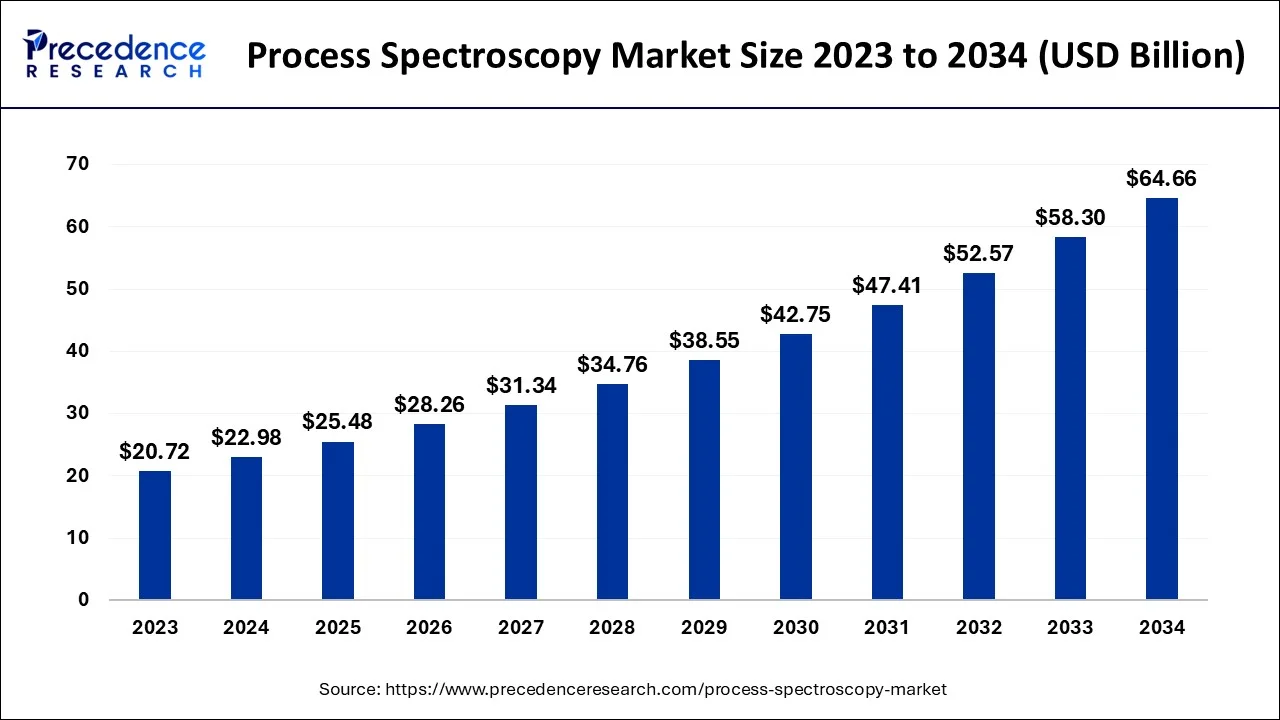
Process Spectroscopy Market Size and Forecast 2024 to 2034
The global process spectroscopy market size was valued at USD 22.98 billion in 2024 and is predicted to achieve around USD 64.66 billion by 2034, poised to grow at a CAGR of 10.90% during the forecast period from 2024 to 2034.
Process Spectroscopy Market Key Companies
- Yokogawa Electric Corporation
- Thermo Fisher Scientific, Inc.
- Shimadzu Corporationth
- Sartorius AG
- Kett Electric Laboratory
- Kaiser Optical Systems, Inc.
- HORIBA Ltd.
- Foss
- Danaher Corporation
- Bruker
- Agilent Technologies, Inc.
Process Spectroscopy Market Recent Developments
- In November 2024, Radiant Vision Systems, known as a world-class manufacturer of light and color measurement systems based on images, introduces a new ProMetric® I16-G-SC, which is an imaging colorimeter and spectrometer combined in one compact device for versatile color measurement solutions.
- In October 2024, APL Photonics presented an original design of an integrated photonic spectrometer, which is based on the imaging of light propagation in MMI waveguides, enhanced by machine learning. Therefore, this work applies to solar spectrum analysis and the detection of particles in optofluidic systems.
- In October 2024, a high-performing spectrometer capable of measuring light at a resolution of 0.05 nanometers, which is 1.6 million times smaller than the width of a human hair was developed by researchers at UC Santa Cruz. The gadget appeared in the paper in IBM APL Photonics which is one of the renowned journals in the field.
- In June 2024, Thorlabs, a contemporary physics research and instrument manufacturing company, announced a new product Surfaceâ€Enhanced Raman Spectroscopy (SERS) Substrate, which is a very sensitive, nondestructive method that can detect lower sample concentrations with lower incident power than the conventional Raman spectrometric techniques. This substrate has a broad application range from analyzing chemical mixtures, identifying substances, quality control, and tracking changes in chemical transformations.
We’ve prepared a service to support you. please feel free to contact us at sales@precedenceresearch.com | +1 804 441 9344