AI in Clinical Trials Market Will Grow at CAGR of 28% By 2033
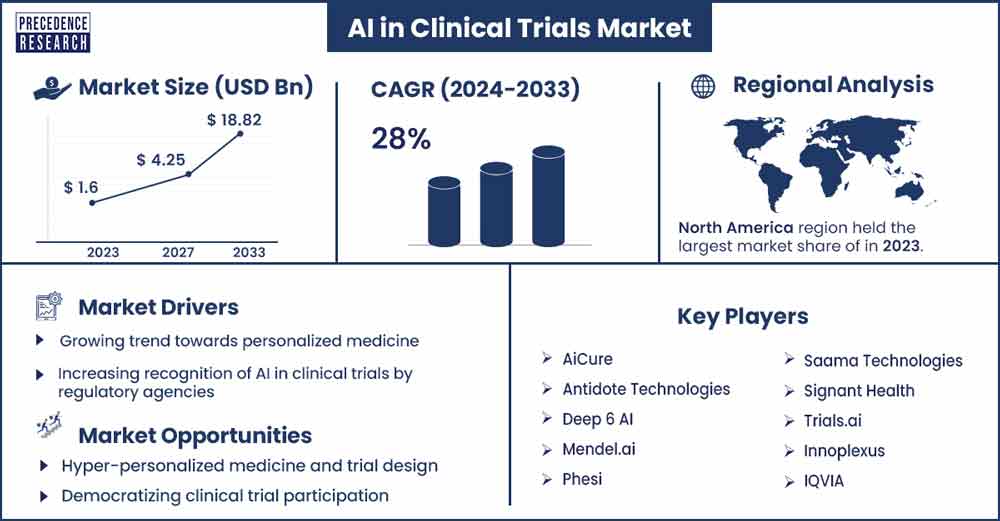
The global AI in clinical trials market size was exhibited at USD 1.6 billion in 2023 and is anticipated to touch around USD 18.82 billion by 2033, expanding at a CAGR of 28% from 2024 to 2033.
Market Overview
Artificial intelligence (AI) stands as the most revolutionary technology of our era in drug development. The AI in clinical trials market encompasses the creation, application, and commercialization of AI technologies, all aimed at enhancing the efficacy, precision, and efficiency of clinical research. The integration of AI in clinical trials has the potential to completely transform the drug development process, enabling more efficient data management, improved decision-making, and overall success of the clinical trial value chain.
AI unlocks the power of advanced analytics, enables automation, and significantly increases speed across all clinical trial phases. The AI in clinical trials market provides pharmaceuticals and biotechnology companies with cutting-edge services such as optimization of protocol design, machine learning (ML) guided analysis of regulatory documents, such as investigational new drug (IND) applications and safety reports, planning of patient recruitment and retaining strategies, real-time monitoring, prediction models of intervention response, etc.
- In October 2023, MyTrialsConnect, a platform for clinical trial recruitment and engagement driven by artificial intelligence, was introduced by Elligo Health Research in collaboration with Avallano. Patients can sign up on the site to participate in the study and become members of a healthcare provider's network. Following enrollment, individuals will be notified by chatbot-based surveys and an automated evaluation of their medical records if they are qualified for a clinical trial.
- In 2023, most healthcare executives relied on artificial intelligence to find and enroll people for clinical trials, cutting down on the time and cost currently necessary, according to a survey conducted by UPMC's Center for Connected Medicine (CCM).
Regional Snapshot
North America is dominant in the AI in clinical trials market during the forecast period. The advanced and well-established healthcare infrastructures in the U.S. and Canada make them favorable for implementing AI in clinical trials. The availability of sophisticated medical imaging equipment, electronic health records (EHRs), and other data sources provide a solid basis for using AI in clinical research processes. It is open to new ideas that can boost productivity, cut expenses, and improve patient results.
An increasing understanding of the possible advantages drives the need for AI applications in clinical trials. North American regulatory agencies, like Health Canada and the U.S. Food and Drug Administration (FDA), have demonstrated a readiness to adjust to technology developments in the healthcare industry. Through standards and regulatory frameworks, they facilitate the use of AI in clinical trials, which incentivizes the sector to invest in and use these technologies.
An increasing understanding of the possible advantages drives the need for AI applications in clinical trials. North American regulatory agencies, like Health Canada and the U.S. Food and Drug Administration (FDA), have demonstrated a readiness to adjust to technology developments in the healthcare industry. Through standards and regulatory frameworks, they facilitate the use of AI in clinical trials, which incentivizes the sector to invest in and use these technologies.
AI in Clinical Trials Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 1.6 Billion |
| Projected Forecast Revenue by 2033 | USD 18.82 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 28% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
AI's ability to process and analyze complex data
The healthcare sector produces significant data from various sources, such as genetic data, medical imaging, and patient records. Conventional techniques for analyzing data can be laborious and may need help to successfully reveal trends or insights from this enormous amount of data. Large, complicated datasets are areas where AI shines, especially regarding machine learning methods. It can swiftly examine a variety of data kinds, spot trends, and draw insightful conclusions.
AI can expedite the medication research and discovery process in setting clinical trials. It can efficiently and effectively process and integrate data from many sources, such as test results, patient demographics, and electronic health records. This contributes to developing a thorough and cohesive picture of the patient category driving the AI in clinical trials market.
- In January 2024, With Quant Health, an AI-powered clinical trial designing business that simulates clinical trials in the cloud to help biotech and pharmaceutical firms develop treatments for patients more quickly and affordably, Accenture stated that it has invested in strategic importance via Accenture Ventures.
Streamlining patient recruitment and retention
A great deal of patient data can be swiftly analyzed by AI technologies, which speeds up the process of finding qualified applicants. Doing so accelerates the hiring process, saving substantial time and other resources. AI makes it possible to implement individualized engagement tactics, adjusting interventions and messages to each patient's unique needs. This leads to improved trial protocol adherence, patient experience, and higher retention rates. Effective patient acquisition and retention reduce waiting times and guarantee that clinical studies stay under budget, reducing costs. AI-driven procedures can lower total trial costs and improve resource allocation driving the AI in clinical trials market.
Restraints
High implementation costs
Adopting AI in clinical trial procedures necessitates enormous costs for infrastructure improvement, software development, and technology acquisition. For many firms, the expenses associated with building new solutions, obtaining advanced AI tools, and guaranteeing compatibility with current systems can be prohibitive.
Specialized AI applications are needed for patient recruiting, data analysis, and predictive modeling in clinical trials. These apps are more expensive to develop and implement overall because they frequently require cooperation from data scientists, technology specialists, and healthcare providers. The cost is also increased by regular upkeep, instruction, and updates. Thus, significantly limiting the adoption services of the AI in clinical trials market.
Stringent regulatory environment
Healthcare authorities have strict regulations and compliance requirements in place that AI applications in clinical trials must follow. It can be difficult and time-consuming to make sure AI systems adhere to these criteria, which causes delays in the creation and application of AI solutions for clinical trials. Sensitive patient data is involved in clinical trials, and regulatory agencies have strict restrictions about gathering, keeping, and using this data. The complexity of implementing AI systems that adhere to these data privacy laws increases and may also reduce the adaptability of the services provided by the AI in clinical trials market.
Opportunities
Real-time data analysis and risk prediction
Due to real-time data analysis, researchers and medical professionals can quickly process and understand data as it becomes available during clinical trials. As a result, decisions are made more rapidly and intelligently, allowing for rapid modifications to patient care, treatment plans, and study protocols. Clinical trial designs can be optimized by AI-powered technologies that analyze past data, spot possible problems, and recommend improvements. This may result in more productive and economical studies, which would cut down on the time and money needed for medication development providing lucrative opportunities for businesses in the AI in clinical trials market.
- In October 2023, a recent World Health Organization (WHO) report outlined important regulatory factors regarding artificial intelligence in the healthcare industry. The article strongly emphasizes determining the efficacy and safety of AI systems, providing appropriate systems to individuals in need as soon as possible, and encouraging communication among stakeholders.
Recent Developments
- In June 2023, in addition to introducing the debut of its unified framework of SaaS-based products to complement its current array of customized solutions and services, Saama expedited the development of clinical trials and commercialization.
- In June 2023, the computational imaging startup Altis Labs, Inc., which uses artificial intelligence to speed up clinical studies, reported that its $6 million US seed fundraising had closed.
- In February 2023, the technology and management consulting company ZS disclosed that it purchased Trials.ai, a pure-play AI and analytics business focusing on healthcare. According to ZS, the purchase is an attempt to support the clinical trials team in improving participant experience and expediting the delivery of treatments to market.
Key Market Players
- AiCure
- Antidote Technologies
- Deep 6 AI
- Mendel.ai
- Phesi
- Saama Technologies
- Signant Health
- Trials.ai
- Innoplexus
- IQVIA
- Median Technologies
- Medidata
Market Segmentation
By Offering
- Software
- Services
By Technology
- Machine learning
- Deep learning
- Supervised
By Application
- Cardiovascular
- Metabolic
- Oncology
- Infectious diseases
By End-user
- Pharma
- Biotech
- CROs
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3743
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308