Enteral Feeding Devices Market Revenue To Attain USD 5.8 Bn By 2032
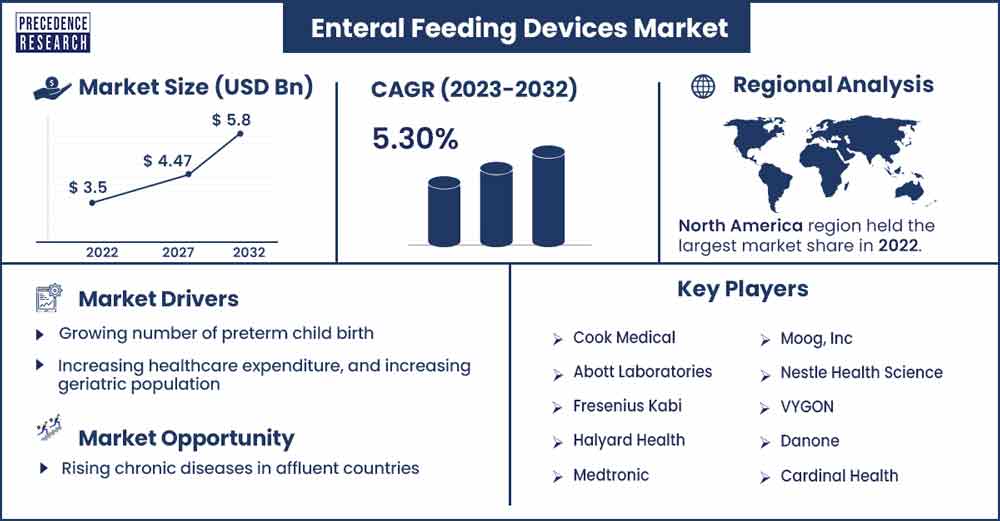
The global enteral feeding devices market revenue surpassed USD 3.5 billion in 2022 and is projected to attain around USD 5.8 billion by 2032, growing at a CAGR of 5.30% from 2023 to 2032.
Market Overview
The enteral feeding device is generally used in the healthcare sector to provide nutrition to patients who are not able to obtain nutrition by mouth or are unable to eat or swallow and need a nutritional supply. Insertion of feeding tube may be for short period for acute conditions or could be lifelong depending upon individuals' health state and need according to the symptoms. Conditions such as premature birth, failure to thrive, digestive disorders, and neurologic and neuromuscular disorder makes patients unable to swallow.
Feeding tubes are excellent options for providing nutrition to patient who are critically brain dead but are on ventilation in the intensive care unit ICU, with their addressed diagnosis. Eternal tubes are a boon for children with premature birth until they become able to eat their own. That is why feeding tubes have gained popularity for many years. People with advanced dementia who need feeding assistance also rely on feeding tubes. Also, people who have gastrointestinal surgery often have a feeding tube until their recovery, which leads through the nose and down to the small intestine.
- According to recent statistics, over 3 million people depend upon feeding tubes to survive globally. The enteral feeding market was valued at around 5.1 billion USD in 2020. And they are expected to grow further in recent years.
Enteral feeding tubes have extensive uses in healthcare sectors like internal care units ICU, critical care units CCU, operation theatres OT and even for home care setups. Furthermore, F.D.A has closely monitored research and development for feeding tubes, and their after-approval effects have been recorded to reduce any complications or misconnections of tubes for patients. To ensure regulatory safety standards, enteral devices need to undergo preprocessing to get approved by the FDA for commercial application of the product in the market in order to limit the chances of risks and injuries associated with feeding devices.
In the USA, regulations have been defined under section 5(b) of the ‘Orphan Drug Act”, which states that a food or supplement which is formulated to be consumed by patients having distinctive diseases or conditions by using enteral feeding and which is done under close supervision of a physician to meet the nutritional requirements and solely based upon recognized scientific proofs are established by medical evaluation.
Regional Snapshot
North America is the dominating region for the enteral feeding devices market. The significant market share is attributed to many factors, such as increasing demand for home care settings, growing premature birth rate, and constant rate of chronic ailments. Approximately 380,000 babies are born prematurely every year, and it's the main cause of baby death. Such health concerns can be resolved by using feeding devices, this actuates the adoption rate for feeding devices in the US market.
Asia Pacific is observed to witness the fastest expansion due to increased awareness of early diagnosis and treatments. China is the leading provider of several enteral feeding devices in the Asia Pacific due to its increasing rate of premature birth and high expenditure on the healthcare sector and new product launches. For instance, in October 2020, Nestle Health Science launched a ready-to-eat protein-based food for malnourished patients precisely fabricated for medical feeding purposes.
Enteral Feeding Devices Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 3.67 Billion |
| Projected Forecast Revenue by 2032 | USD 5.8 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 5.30% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Prevalent cases of premature birth
Pre-mature birth rates have become a major concern related to infants as premature babies with a weight less than 1500gm need more critical care and sufficient feeding to support further growth. As premature babies are unable to swallow, suckle, or breathe on their own, they need external support along with observations.
Moreover, infants born with genetic disorders require feeding tubes in order, to get nutritional support and to survive. Feeding tubes are designed specifically for newborns who need feeding support and are much safer to use. As a result, enteral feeding tubes have become crucial to treat patients or infants with critical health conditions. That will further show medical staff's interest in using feeding tubes and leading a market at a higher pace, in turn driving its total revenue share globally.
Restraint
Lack of awareness for enteral device
General understanding and precautionary practice are crucial to placing and handling enteral feeding devices as they will be directly placed into the internal intestinal part, which is very sensitive to foreign intervention. Although these products are gaining popularity in the medical field, they still need to be understood by patients and even professionals, which may lead to other health complications like metabolic irritation or infection. Although these are not severe conditions to not take over, they will exacerbate patient health, burdened with additional costs for treatment later. Such factors may hamper the demand for feeding devices and restrain the market's growth on a global scale.
Opportunity
Rising chronic diseases in affluent countries
Emerging regions like Asia Pacific are projected to fuel market growth further, including potential opportunities for new entrance into the feeding device market. This is because rising economies like India and China are primarily focusing on research and development with innovative medical devices with cutting-edge technology to provide reliable medical practice for patients dealing with chronic diseases. Cancer is one of the most growing frequent diseases globally. As chronic diseases need to provide long-term treatment, medical faculty also needs to take care of nutritional therapies to gain vital elements to the body and make it recover faster. These necessities further lead to increased demand for enteral feeding devices in the market and are projected to propel market growth in every possible way on a broader scale.
Recent Developments
- In November 2022, Amsino Medical Group Enterprise made an announcement that it had gained 510(k) approval for its new product name, ‘AMSafe NeuFlo needleless connector’, which will be compatible with enteral feeding devices.
- In March 2022, Macatt Medica, a leading distributor in Peru, was acquired by Vygon from France. The acquisition is believed to establish the presence of Vygon in the South American region to provide various feeding devices on a broader range.
- In November 2022, to fight against infant mortality and malnutrional risk factors for premature birth, Medela AG from the US made a partnership with Ronald Mcdonald House Charities, which is US a nonprofit organisation. This collaboration aims to provide NICU supplies, which is an integral part of its breastfeeding business unit and support its 60 programs worldwide.
Major Key Players
- Cook Medical
- Abott Laboratories
- Fresenius Kabi
- Halyard Health
- Medtronic
- Moog, Inc
- Nestle Health Science
- VYGON
- Danone
- Cardinal Health
- Braun
- Boston Scientific
- R. Bard
- Conmed Corporation
Market Segmentation
By Product Type
- Administration Sets
- Enteral Feeding Pump
- Enteral Syringe
- Enteral Feeding Tube
- Others
By Age Group
- Pediatrics
- Adult
By Application
- Gastrointestinal Disease
- Cancer
- Malnutrition
- Neurological Disorder
- Others
By End User
- Ambulatory Surgical Center (ASCs)
- Hospital
- Home Care
- Others
Buy this Research Report@ https://www.precedenceresearch.com/checkout/1226
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308