In-vitro Inflammatory Bowel Disease Diagnostics Market Revenue to Attain USD 2.86 Bn by 2033
In-vitro Inflammatory Bowel Disease Diagnostics Market Revenue and Trends 2025 to 2033
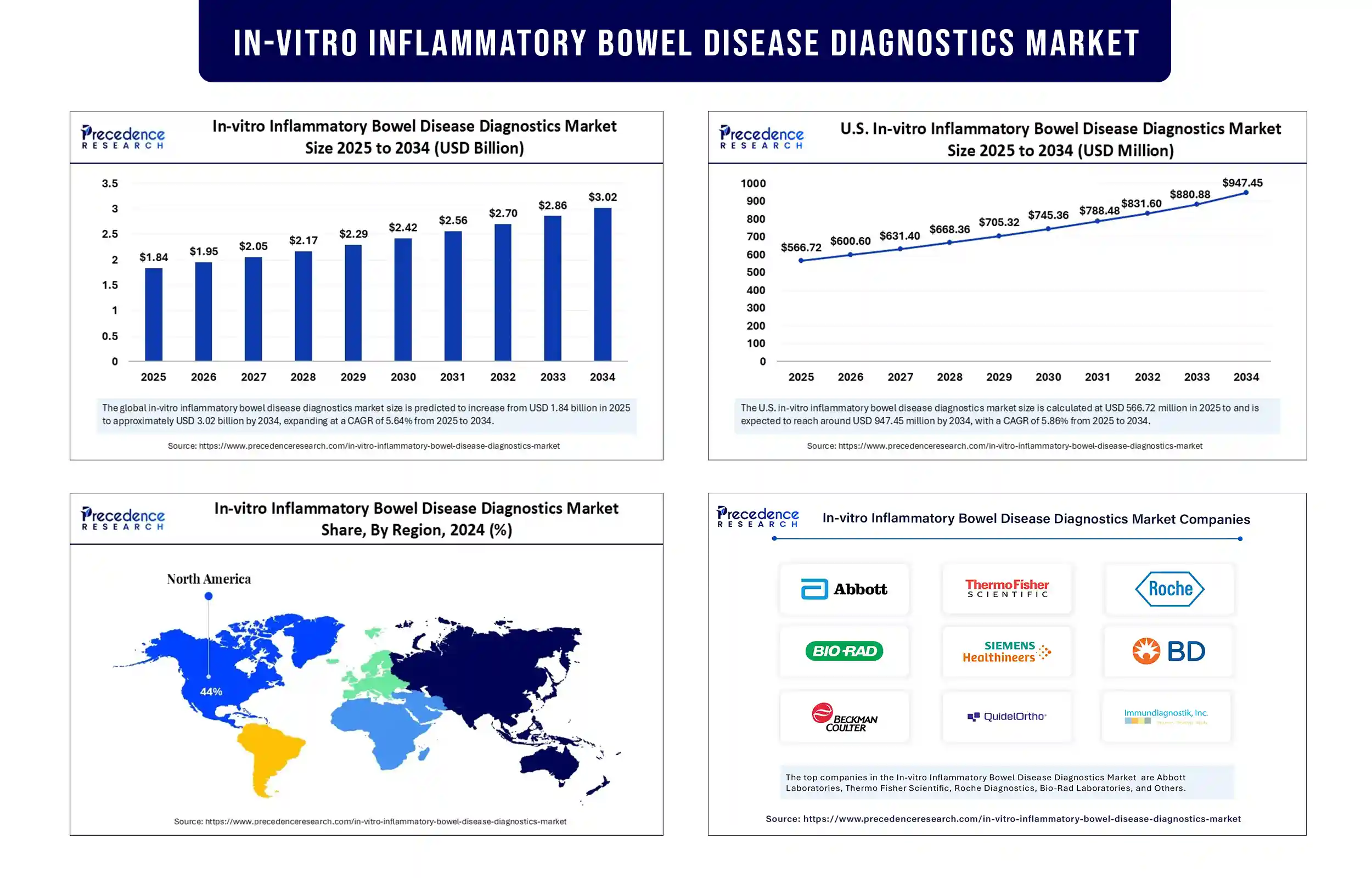
The global in-vitro inflammatory bowel disease diagnostics market revenue surpassed USD 1.84 billion in 2025 and is predicted to attain around USD 2.86 billion by 2033, growing at a CAGR of 5.64%. The growth of the in-vitro inflammatory bowel disease diagnostics market is growing due to the global increase in IBD prevalence, combined with the rising demand for non invasive, biomarker‐based, and point‐of care diagnostic tools that enable earlier, more accurate detection and personalized disease management.
Market Overview
The worldwide in-vitro inflammatory bowel disease diagnostics market is poised for remarkable growth, driven by the increasing global prevalence of inflammatory bowel diseases, which includes Crohn's disease and ulcerative colitis. The increasing awareness of gastrointestinal health, advancements in non-invasive diagnostics, and increased availability of biomarker testing are expected to drive the growth of the market in the coming years. Rising government initiatives, supporting healthcare investment, and higher research & development funding for creating novel diagnostic tools for early and accurate detection of disease are also contributing to market growth.
Segment Insights
- By test type, the biomarker tests segment dominated the market in 2024. This is due to their non-invasiveness and superior diagnostic accuracy. The increased acceptance of biomarker-based tests amongst physicians and patients bolstered the growth of the segment.
- By disease type, the Crohn's disease segment led the market in 2024. This is primarily due to the growing demand for accurate and non-invasive diagnostics, as well as the increasing prevalence of Crohn’s disease.
- By sample type, the stool segment dominated the market, accounting for the largest share in 2024. This is mainly due to its effectiveness in distinguishing IBD. The increased preference for non-invasive fecal biomarker tests to diagnose and monitor inflammatory bowel disease has compounded this preference when assessing fecal biomarkers like calprotectin versus serum inflammatory bowel disease biomarkers.
- By end-user, the hospitals segment led the in-vitro inflammatory bowel disease diagnostics market in 2024. This is primarily because hospitals offer a suite of diagnostic services, attracting more patients.
- By technology, the immunoassays segment maintained dominance in the market in 2024. This is mainly due to their advantages for testing, includes their diagnostic accuracy, speed, ease of use, and reliability in detecting clinically important biomarkers that indicate disease.
Regional Insights
North America registered dominance in the in-vitro inflammatory bowel disease diagnostics market in 2024. This is mainly due to its advanced healthcare systems, reimbursement policies, and the increased rates of Crohn's disease and ulcerative colitis cases. The U.S. and Canada are major players in the market due to the rising demand for advanced diagnostic solutions and the widespread adoption of biomarker testing.
Meanwhile, Asia Pacific is expected to grow at the fastest rate in the coming years, driven by the rising prevalence of IBD. China, India, Japan, and South Korea are leading the charge due to rising healthcare expenditures, growing awareness of IBD, and the availability of non-invasive biomarker testing.
In-vitro Inflammatory Bowel Disease Diagnostics Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.84 Billion |
| Market Revenue by 2033 | USD 2.86 Billion |
| CAGR from 2025 to 2033 | 6.64% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development
- In October 2024, PathAI announced a partnership with the Crohn’s & Colitis Foundation. The collaboration will empower academic and biopharmaceutical researchers to unlock insights into inflammatory bowel disease (IBD) through the unique combination of quantitative histopathology data and well-characterized multi-modal clinical and molecular data.
(Source: https://www.pathai.com)
In-vitro Inflammatory Bowel Disease Diagnostics Market Key Players
- Abbott Laboratories
- Thermo Fisher Scientific
- Roche Diagnostics
- Bio-Rad Laboratories
- Siemens Healthineers
- Becton, Dickinson and Company (BD)
- Danaher Corporation (Beckman Coulter)
- QuidelOrtho Corporation
- Calpro AS
- Immundiagnostik AG
- TechLab, Inc.
- Prometheus Biosciences (a part of Merck)
- Epitope Diagnostics, Inc.
- Myriad Genetics
- Genova Diagnostics
- Biomerica Inc.
- Eurofins Scientific
- GenoSensor Corporation
- Alfa Scientific Designs, Inc.
- F. Hoffmann-La Roche Ltd.
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6468
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344