Multiple Myeloma Market 2025 Set to Grow with Innovative Treatments and Expanding Access
Multiple Myeloma Market Size, Shares, Trends and Key Players
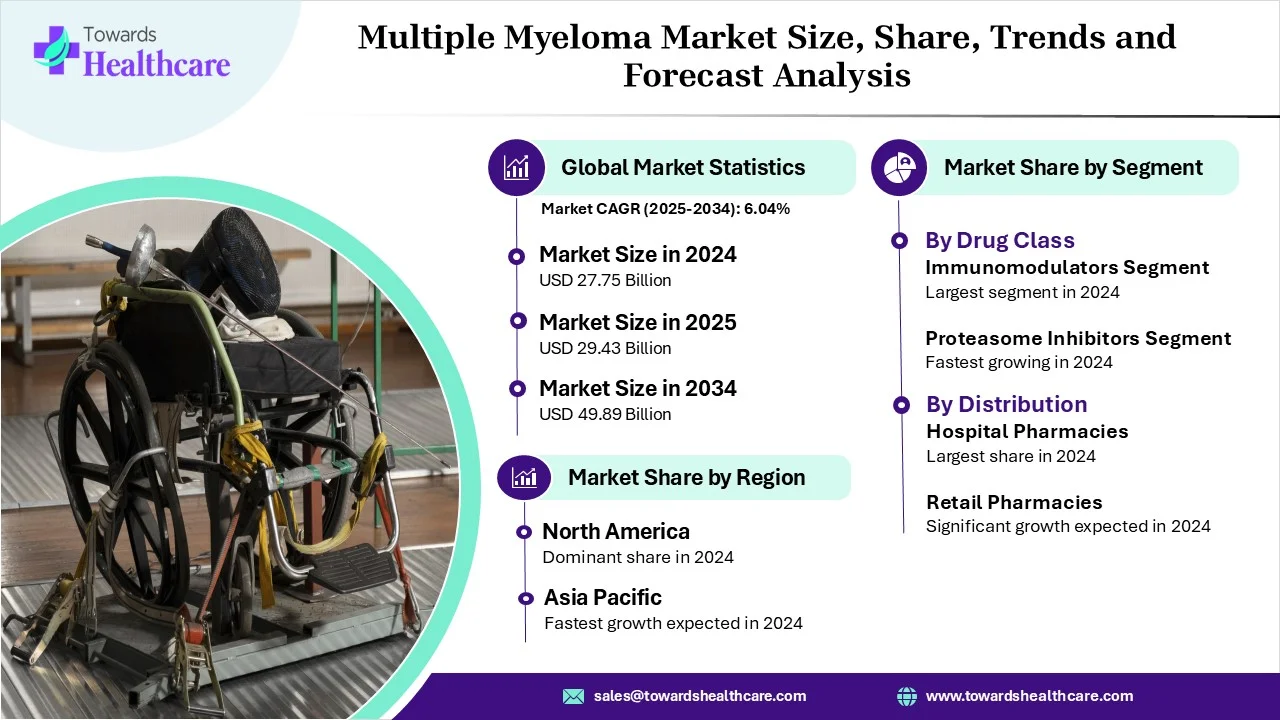
According to Towards Healthcare study, the global multiple myeloma market size was estimated at USD 27.75 billion in 2024 and is anticipated to reach around USD 49.89 billion by 2034, growing at a CAGR of 6.04% from 2025 to 2034. The symptoms of blood cancer and abnormal cellular tissue drive the need for effective treatments against certain health conditions.
Global Outlook
Multiple myeloma is caused by a plasma cell in the bone marrow and is a type of blood cancer. The U.S. FDA approvals for certain medicines expanded initial treatment options for multiple myeloma. The National Cancer Institute (NCI) and many other research institutes in India work to advance research on multiple myeloma and boost patient care through funding and clinical trials.
Market Opportunity
The Multiple Myeloma Research Foundation (MMRF) is the largest non-profit organization in the U.S. which drives patient care. The various government programs such as Medicare, Centers for Medicare, and Medicaid Services, Healthcare.gov, State Health Insurance Program for Seniors, Health Insurance Resource Center, and State Insurance Information assist with financial benefits, health insurance, and prescription coverage. The financial resources for plasma cell myeloma are HealthWell Foundation, Leukemia and Lymphoma Society (LLS) Co-Pay Assistance Program, Local Financial Assistance Programs, Co-Pay Relief Program Fund, PAN Foundation, Patient Aid Program, Urgent Need Programs, Patient Advocate Foundation (PAF), and Triage Cancer.
Key Growth Factors
- Advances in plasma cell myeloma research include immunotherapy, Bispecific T-Cell Engagers, Immunomodulating Drugs, targeted therapies, stem cell transplant research, and NCI supported research programs.
- Research on pathophysiology, treatment, patient care strategies, and breakthroughs in plasma cell myeloma drives the growth of the multiple myeloma market.
Market Restraint
With advancements in the oncology field, treatments for multiple myeloma are becoming increasingly complex. There is an emerging need for improved sequencing strategies and novel therapies due to the rise of triple-class refractory disease. Minimal Residual Disease (MRD) assessment and B-cell maturation antigen (BCMA) therapies are two potential solutions to these issues but also impact their uptake.
Segmental Outlook
Drug Class Insights
The immunomodulators segment dominated the multiple myeloma market in 2024 owing to direct anti-cancer effects, immune system modulation, and benefits in clinical use. They induce cell death, prevent cell proliferation, and reduce anti-apoptotic proteins. They enhance T-cell activity, activate natural killer cells, and decrease immunosuppression.
The proteasome inhibitor segment is expected to grow at the fastest CAGR in the multiple myeloma market during the forecast period due to its major role in inducing cell death, inhibiting cell adhesion, and eliciting anti-angiogenic effects. It improves bone health and remains effective in the diagnosis and treatment of certain health conditions. It improves the overall survival of patients and provides significant clinical benefits.
Distribution Insights
The hospital pharmacies segment dominated the multiple myeloma market in 2024 owing to the optimized medication management and enhanced patient education. They improve supportive and preventive care and drive optimized coordination and efficiency. They focus on reducing medication errors and managing side effects.
The retail pharmacies and drug stores segment is expected to grow at the fastest CAGR in the multiple myeloma market during the forecast period due to patient education and counseling, adverse event monitoring, and counseling on supportive medications. They help to access therapies and provide financial and logistics support. They offer convenience, enable collaboration with care teams, and empower patients.
Geographical Outlook
North America
North America dominated the multiple myeloma market in 2024 driven by developed healthcare infrastructure, high healthcare expenditure, and innovative therapeutic options. The International Myeloma Foundation (IMF) located in the USA keeps an eye on new treatments and research on plasma cell myeloma. The American Society of Clinical Oncology (ASCO) and European Hematology Association (EHA) focus on delivering the newest and more innovative approaches to myeloma treatments. The Center for Cancer Research, an internal cancer center of NCI, works to improve the lives of cancer patients through clinical trials researching drug therapy.
U.S.
According to the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute, the U.S. accounts for 36,110 estimated new cases of multiple myeloma in 2025. Plasma cell myeloma represents 1.8% of all new cancer cases in the U.S. There are several new government-approved treatments and major non-governmental research programs which drive the growth of the U.S. healthcare sector.
Asia Pacific
Asia Pacific is expected to grow at the fastest CAGR in the multiple myeloma market during the forecast period due to advancements in treatment and diagnostics as well as improvements in healthcare and infrastructure. The Asian Myeloma Network (AMN) contributed to the development of its own clinical trial network to allow early access to drugs in Asian countries and conduct clinical trials relevant to Asians.
In February 2025, the Ministry of Health, Labor and Welfare (MHLW) in Japan approved sarclisa for treating adult patients who are dealing with plasma cell myeloma.
India
In March 2025, the Postgraduate Institute of Medical Education and Research (PGI) launched clinical trials for CAR T-cell therapy in plasma cell myeloma. The Indian government made efforts to promote research, development, and deployment of CAR T-cell therapy.
Strategic Moves by Key Players
- In June 2025, Johnson & Johnson announced the Committee for Medicinal Products for Human Use (CHMP) approval for DARZALEX to treat patients with high risk of plasma cell myeloma.
- In May 2025, CellCentric secured a $120 million investment to advance inobrodib for the treatment of plasma cell myeloma.
Market Companies
- Bristol Myers Squibb
- Takeda Pharmaceutical Company Ltd.
- Johnson & Johnson
- Novartis
- Amgen Inc.
- AbbVie Inc.
- Sanofi S.A.
- F.Hoffman-La Roche Ltd.
- Genentech
- Legend Biotech Corporation
- Adaptive Biotechnologies Corporation
Segments covered in the report
By Drug Class
- Immunomodulators
- Proteasome Inhibitor
- Anti-CD38 Monoclonal Antibody
- Alkylating Agents
- Others
By Distribution
- Hospital Pharmacies
- Retail Pharmacies & Drug Stores
- Online Pharmacies
By Region
- North America
- U.S.
- Canada
- Mexico
- Asia Pacific
- China
- Singapore
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Source: https://www.towardshealthcare.com/insights/multiple-myeloma-market-sizing