Non-Invasive Prenatal Testing Market is Likely to Rise at 12.1% CAGR By 2032
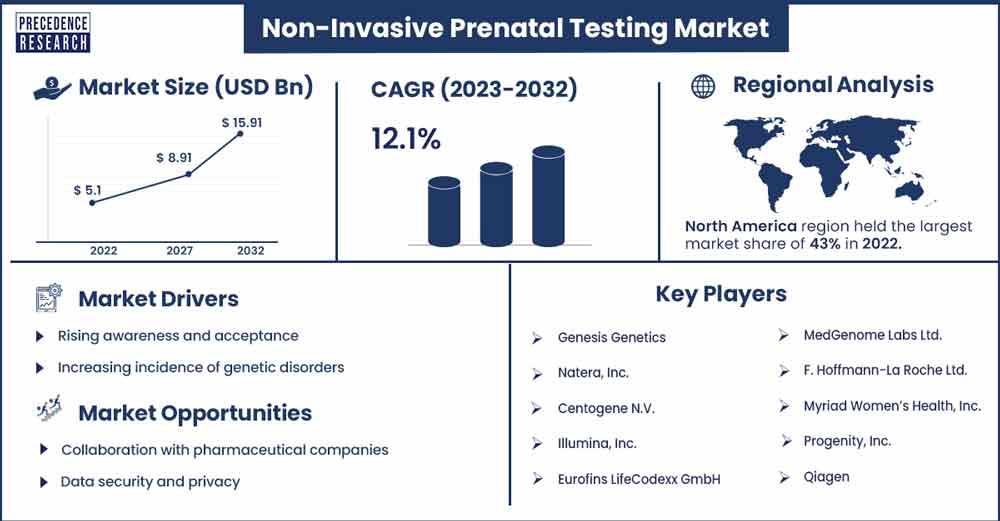
The global non-invasive prenatal testing market size was exhibited at USD 5.1 billion in 2022 and is projected to attain around USD 15.91 billion by 2032, growing at a CAGR of 12.1% during the forecast period 2023 to 2032.
Market Overview
Non-invasive prenatal testing (NIPT) is a screening technique that examines cell-free DNA (cfDNA) in the mother's blood. It is used to determine the likelihood of the fetus having chromosomal abnormalities such as Down syndrome, Edwards syndrome, or Patau syndrome. NIPT has demonstrated superior sensitivity and accuracy over conventional prenatal screening techniques and has a reduced risk of miscarriage than invasive techniques like amniocentesis or chorionic villus sampling (CVS).
Pregnant patients and healthcare professionals looking for more dependable outcomes have increased usage. The accuracy and dependability of NIPT have been enhanced by ongoing technological developments in DNA sequencing and bioinformatics, which has increased its popularity. NIPT's uses are growing beyond chromosomal abnormalities as it is rapidly being applied to detecting various genetic illnesses and ailments. Higher acceptance rates result from more significant knowledge about the advantages of non-invasive prenatal testing among expectant mothers and medical professionals.
There is a correlation between an increased risk of chromosomal abnormalities and the trend of postponing childbearing and growing the mother's age. A higher chance of chromosomal abnormalities in the fetus is linked to older mothers. The need for sophisticated, non-invasive prenatal testing techniques like NIPT rises as more women put off becoming pregnant, promoting the growth of the market.
- In November 2022, Redcliff Labs announced its intention to contribute an additional $10 million to diversifying its genetic and specialized testing services. It previously invested $6 million in genetics and specialized testing covering everything from prenatal to oncology, neurology, nephrology, gut microbiome, and the fastest-growing field of pharmacogenomics and is now focused on its AI platform for next-generation diagnostic technologies.
- In March 2022, aiming to develop accessible, powerful, and accurate molecular diagnostics, Billiontoone, Inc. announced the closing of a $125 million Series C fundraising round that was oversubscribed and included leading international investors.
Regional Snapshot
There has been a notable surge in the NIPT market worldwide; however, North America holds the largest share. The precision and dependability of NIPT have increased due to ongoing developments in bioinformatics and DNA sequencing technology. There is an increased frequency of chromosomal abnormalities brought on by the tendency to postpone delivery. Thus, older pregnant women and medical professionals need to have heightened knowledge of the advantages of NIPT.
Because non-invasive prenatal testing is more accurate than traditional screening procedures and more people are becoming aware of it, the market for this type of testing has been expanding rapidly in the US. The range of disorders that can be detected is developed, and ongoing developments improve the accuracy of NIPT in bioinformatics and sequencing technology. For several chromosomal anomalies, the FDA has approved certain NIPT products; therefore, adherence to laws is essential for businesses operating in this sector. NIPT is now more widely available to a larger population due to increases in insurance coverage over time. Future developments in bioinformatics and sequencing technology could boost NIPT's accuracy and applicability, increasing its adoption rate.
North America has several key players in the NIPT market, including but not limited to F. Hoffmann-La Roche Ltd., Natera, Inc., Centogene N.V., Illumina Inc., Genesis Genetics, Eurofins LifeCodexx GmbH, Myriad Women's Health, and Inc MedGenome Labs Ltd.
- In July 2022, AI intelligent quantitative products have been launched by Genesis. Genesis employs innovative AI quantitative products to use artificial intelligence technology across multiple domains, including logic mathematics, linear analysis, statistical psychology, and other professional disciplines. This allows this system to be quantitatively analyzed, and risks and costs can be predicted.
- In August 2022, as a part of a strategic fundraising round headed by Novo Holdings, a health sciences investor, Medgenome raised $50 million; other current investors, including Leapfrog Investments and Sofina, also participated in the round. With the money, the business plans to reach Africa, the Middle East, and South Asia.
Modifying regulations and rules concerning prenatal testing may affect NIPT's acceptance and accessibility in Canada. Growing public and healthcare professional knowledge of NIPT's advantages may explain why it's becoming more widely used. The Canadian healthcare system's infrastructure, which includes the availability of qualified personnel and the adoption of new technology, may impact the accessibility and availability of NIPT. The regulations and coverage of public and commercial health insurance reimbursement can significantly impact how affordable and easily accessible non-invasive prenatal testing is for expectant mothers.
- In August 2023, it was disclosed that Nuchem Sciences, located in Canada, is to be acquired by Sygnature Discovery. With this acquisition, Sygnature's position as a significant global provider of comprehensive drug discovery phase solutions solidified, and its goal of dominating the global market was furthered.
Non-Invasive Prenatal Testing Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 5.69 Billion |
| Projected Forecast Revenue by 2032 | USD 15.91 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 12.1% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Early detection
With the shift from old-fashioned techniques to next-generation sequencing (NGS), the NIPT sector has seen notable breakthroughs in sequencing technologies. Identifying common trisomies is no longer the exclusive use for NIPT; its scope has grown over time. These days, several tests additionally check for anomalies related to the sex chromosome and microdeletions. This enhanced service improves the ability to identify genetic disorders early in gestation. Businesses are always trying to increase the sensitivity and specificity of their tests, and by giving end consumers more options, this competition helps them.
Rising awareness and acceptance
When it comes to identifying common chromosomal anomalies like Down syndrome, Edwards syndrome, and Patau syndrome, NIPT has proven to be highly accurate. The increased adoption of NIPT among healthcare professionals and expectant parents can be attributed to its reliability. Because NIPT is non-invasive, it is a desirable choice for people who wish to minimize risks while still gaining crucial genetic information about the fetus. The likelihood of chromosomal abnormalities in the fetus increases with maternal age. Delaying pregnancy is a popular decision among women, and this trend in the population increases the need for sophisticated prenatal screening techniques like NIPT.
Restraints
Limited diagnostic capability
When a fetus has a mixture of standard and aberrant cells, it is called mosaicism, and NIPT may not be able to identify it. Mosaicism occasionally produces false-positive or false-negative results. Minor chromosomal abnormalities (microdeletions and microduplications) may be more complex for NIPT to detect because the test is primarily designed to identify common chromosomal abnormalities. Sometimes, the test finds anomalies in the mother's DNA instead of the fetus's, which might produce false positives. Further testing may be necessary to provide clarification if specific NIPT results are returned as uncertain or have a higher likelihood score.
Informed consent challenges
This pressure may impact the voluntary aspect of consent. Genetic counseling is essential to guarantee that people receive thorough information and support to make educated decisions. The private sector's commercialization of NIPT may result in pushy marketing tactics that highlight the test's advantages while keeping its drawbacks hidden. Expectant parents may have significant psychological and emotional fallout from their NIPT results. Enough knowledge about the possible emotional effects of both favorable and unfavorable outcomes is essential for making well-informed decisions. NIPT was first designed to screen for particular chromosomal defects, but it has now grown to test for a wider variety of genetic disorders.
Opportunities
Expanded test offerings
Businesses and researchers may be trying to broaden the use of NIPT to detect chromosomal abnormalities other than the usual trisomies. Research and technological advancements may make it possible to identify genetic disorders and anomalies earlier in pregnancy, enabling earlier medical interventions or counseling. More thorough genetic information about the fetus, including details regarding single gene abnormalities and other genetic problems, might be provided by NIPT. Even while many NIPT tests can already determine the sex of the fetus, further advancements could improve the precision of this data.
Data security and privacy
It is imperative for healthcare providers and testing facilities to guarantee that patients are informed about the privacy consequences and have the choice to consent or object to any further usage of their data, including research. Sensitive genetic information is transmitted during NIPT. Robust encryption techniques are essential to secure data while it is being shared between testing labs, medical facilities, and other relevant entities. It is necessary to safely keep genetic data in accordance with industry guidelines and laws. Establishing and upholding thorough data protection policies, doing frequent risk assessments, and offering continual staff training are all necessary to comply with these rules.
Recent Developments
- In November 2023, Yourgene Health, a member of the Novacyt group of companies, implemented Morocco's first non-invasive prenatal testing workflow. This process is based on the IONA test. By providing this service locally, the parent company, Laboriad can expand its outreach.
- In January 2023, Juno Diagnostics (JunoDxTM), a company dedicated to providing patients with essential health information by enhancing accessibility, affordability, and transparency, announced the launch of Juno Hazel™ Plus, a non-invasive prenatal screening (NIPT) solution made possible by finger-prick blood samples, and the establishment of an Early Access Program (EAP) to broaden its product portfolio.
Key Market Players
- Genesis Genetics (CooperSurgical, Inc.)
- Natera, Inc.
- Centogene N.V.
- Illumina, Inc. (Verinata Health, Inc.)
- Eurofins LifeCodexx GmbH
- MedGenome Labs Ltd.
- F. Hoffmann-La Roche Ltd. (Ariosa Diagnostics)
- Myriad Women’s Health, Inc. (Counsyl, Inc.)
- Progenity, Inc.
- Qiagen
- Laboratory Corp. of America Holdings
- Quest Diagnostics, Inc.
Market Segmentation
By Component
- Instruments
- Kits and Reagents
- Services
By Application
- Down Syndrome (trisomy 21)
- Edwards Syndrome (trisomy 18)
- Patau Syndrome (trisomy 13)
- Turner Syndrome
- Other Applications
By End User
- Hospitals
- Diagnostic Labs
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3362
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308