DNA Repair Drugs Market Size and Growth 2025 to 2034
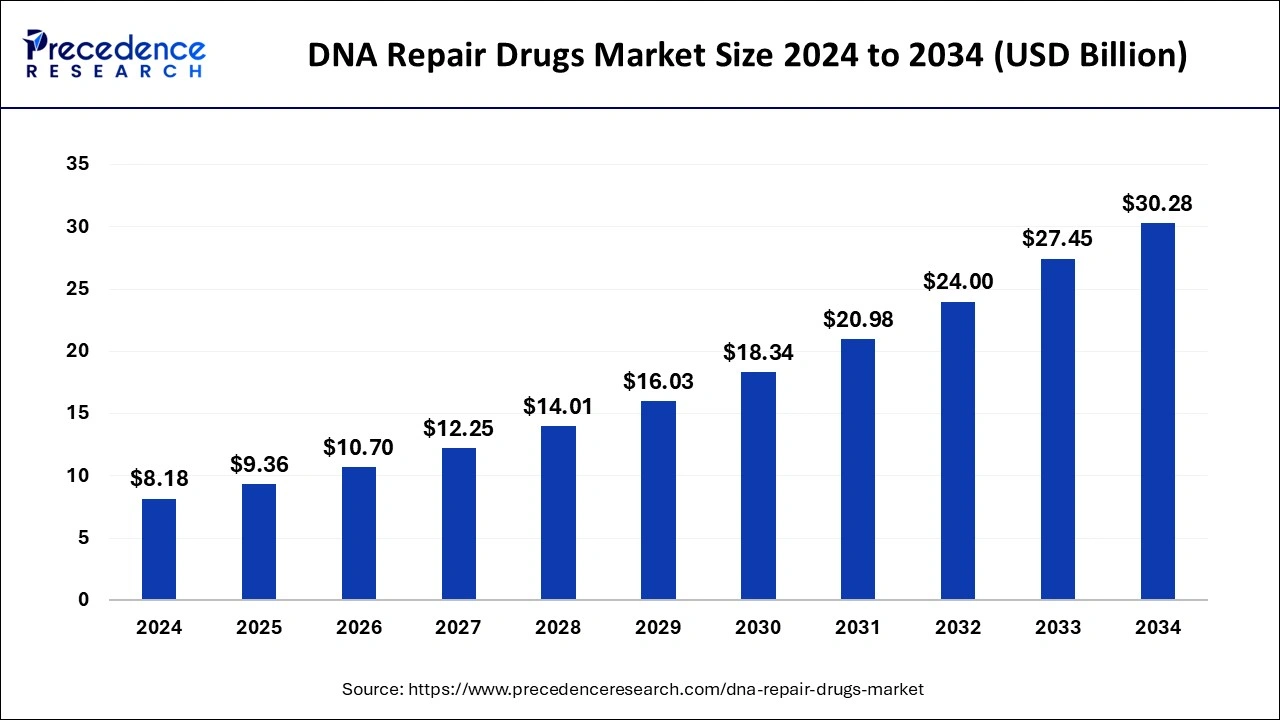
The global DNA repair drugs market size is accounted at USD 9.36 billion in 2025 and predicted to increase from USD 10.70 billion in 2026 to approximately USD 30.28 billion by 2034, expanding at a CAGR of 13.98% from 2025 to 2034. Advancements in precision medicine, DNA repair drug development, tailored treatment to individual genetic profiles, increased understanding of DNA repair deficiencies, and regulatory support are some factors that encourage the growth of the market.
DNA Repair Drugs MarketKey Takeaways
- In terms of revenue, the global DNA repair drugs market was valued at USD 8.18 billion in 2024.
- It is projected to reach USD 30.28 billion by 2034.
- The market is expected to grow at a CAGR of 13.98% from 2025 to 2034.
- North America dominated the DNA repair drugs market in 2024.
- Asia Pacific is projected to emerge as a significant player in the market.
- By product type, the PARP inhibitors segment held the largest share of the market in 2024.
- By product type, the ATM kinase segment is expected to witness the fastest growth during the forecast period.
- By application type, the cancer therapy segment held the dominating share of the market in 2024.
- By application type, the genetic disorders commercial consumers segment is observed to witness a significant rate of expansion during the forecast period.
- By distribution channel, the hospital pharmacies segment held a significant share of the in 2024.
- By distribution channel, the specialty clinics segment is projected to experience substantial growth in the coming years.
Market Overview
DNA repair drugs are a class of pharmaceuticals designed to address abnormalities or deficiencies in the DNA repair mechanisms of cells. DNA repair processes are crucial for maintaining genomic stability, as they correct damage caused by various endogenous and exogenous factors, including radiation, chemicals, and metabolic byproducts. When these repair mechanisms fail, it can lead to the accumulation of mutations and genomic instability, which are associated with cancer and other genetic disorders.
Therapeutics produced by the DNA repair drugs market function by either enhancing the activity of DNA repair enzymes or inhibiting pathways that promote DNA damage. For instance, some drugs target specific repair pathways, such as base excision repair (BER), nucleotide excision repair (NER), or homologous recombination (HR). By modulating these pathways, DNA repair drugs aim to restore the cell's ability to accurately repair DNA damage, thereby preventing or treating diseases associated with genomic instability.
In clinical practice, the DNA repair drugs market finds application primarily in cancer therapy. Tumors with defective DNA repair pathways, such as those with mutations in the BRCA genes, are particularly sensitive to certain DNA repair inhibitors. By exploiting these vulnerabilities, DNA repair drugs can selectively target cancer cells while sparing normal cells, offering a promising approach to personalized cancer treatment. Additionally, ongoing research aims to expand the use of DNA repair drugs beyond cancer therapy, potentially addressing a broader range of genetic diseases characterized by impaired DNA repair mechanisms.
DNA Repair Drugs MarketGrowth Factors
- Enhanced repair mechanisms in faulty DNA targeting drugs facilitate the activation and amplification of cellular mechanisms dedicated to fixing damaged DNA strands, thereby bolstering the repair process.
- Drugs designed for targeted interventions are preferred as they specifically target pathways involved in DNA repair, allowing for precise intervention at the molecular level to address various types of damage.
- Therapies that increase cell viability promote efficient DNA repair. These drugs help maintain the integrity of genetic material within cells, ultimately enhancing cell survival and function.
- Mutation-resistant DNA repair drugs contribute to lowering the occurrence of mutations by promptly identifying and rectifying errors in the DNA sequence, thus safeguarding genomic stability.
- Preventative therapeutics in DNA repair drugs hold promise for mitigating the risk of diseases associated with impaired DNA repair, such as cancer, by bolstering the body's natural defense mechanisms against genetic damage.
Recent Trends
Rising Focus on Cancer Treatment and Precision Medicine:
As cancer cases continue to rise worldwide, the market for DNA repair medications is expanding. These medications are essential to modern oncology because they target defective DNA repair mechanisms that lead to the growth of cancer cells. Targeted DNA repair therapies are becoming more and more popular as a result of precision medicine, which bases treatment plans on genetic profiles.
Dominance of PARP Inhibitors with New Drug Classes Emerging:
Because they are effective in treating BRCA-mutated cancers like breast and ovarian cancer, PARP inhibitors continue to be the most commonly used DNA repair medications. However, the industry is now moving toward novel inhibitors that target treatment resistance and combat a wider variety of tumors, such as ATR, ATM, and DNA-PK. The therapeutic potential of DNA repair techniques is being increased by this diversification.
Growing Popularity of Combination Therapies:
DNA repair drugs are increasingly being used alongside chemotherapy, immunotherapy, and radiation therapy. This combination approach enhances treatment effectiveness and reduces cancer recurrence. Many drugmakers are now conducting trials to explore the benefits of using these agents as part of first-line treatment instead of just late-stage therapy.
Market Outlook
- Industry Growth Overview: As treatments that target DNA damage response (DDR) pathways become more popular in oncology and other fields, the market for DNA repair drugs is expanding rapidly. The need for therapies that take advantage of DNA repair flaws in tumors is being driven by the rise in cancer incidence, developments in genomic diagnostics, and the growth of precision medicine. The market's reach is being expanded by the growing emphasis on combination therapies and first-line maintenance settings.
- Sustainability Trends: From a sustainability perspective, DNA repair drugs promise improved treatment efficiency by enabling more precise therapies, potentially reducing unnecessary treatments and improving outcomes. The integration of companion diagnostics helps tailor therapy, thereby optimizing resource wastage in ineffective treatments. However, high development costs, lengthy regulatory cycles, and the need for advanced diagnostics infrastructure remain challenges to scalable, sustainable development.
- Startups Ecosystem: Alongside the more established PARP class, established pharmaceutical companies and biotech startups are developing the next generation. DDR inhibitors in a thriving innovation ecosystem. To create integrated solutions, these developers are collaborating with genomic and diagnostic companies. The main obstacles facing startups are proving clinical benefit, controlling high R&D costs, and obtaining reimbursement access; however, those that are successful are influencing the direction of targeted cancer therapy.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 13.98% |
| Market Size in 2025 | USD 9.36Billion |
| Market Size in 2026 | USD 10.70 Billion |
| Market Size by 2034 | USD 30.28 Billion |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Drug Type, Application Type, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing incidence of cancer
The rising prevalence of cancer worldwide is a significant driver for the DNA repair drugs market. As cancer incidence continues to escalate due to factors such as aging populations, lifestyle changes, and environmental exposures, there is a growing demand for innovative treatments targeting specific molecular pathways involved in DNA repair. DNA repair drugs offer promising therapeutic options, especially for cancers with defects in DNA repair mechanisms, such as those caused by BRCA mutations.
Advancements in precision medicine
The emergence of precision medicine and personalized cancer therapy has fueled the demand for the DNA repair drugs market. With advancements in genomic sequencing technologies, healthcare providers can identify specific genetic alterations in individual patients' tumors, including mutations affecting DNA repair pathways. This molecular profiling enables the selection of targeted therapies, including DNA repair inhibitors, tailored to the unique genomic characteristics of each patient's cancer. As precision medicine becomes more integrated into clinical practice, the demand for DNA repair drugs is expected to continue growing.
Restraints
Regulatory challenges
One significant restraint in the DNA repair drugs market is the stringent regulatory requirements governing the development and approval of pharmaceuticals. Regulatory hurdles may arise from the need to demonstrate clinical benefit in specific patient populations, such as those with rare genetic disorders or treatment-resistant cancers. Meeting these regulatory requirements poses challenges for drug developers, potentially delaying market entry and limiting the availability of DNA repair drugs for patients in need.
The complex mechanisms involved in DNA repair and the potential for off-target effects necessitate rigorous preclinical and clinical evaluations to ensure safety and efficacy. Regulatory agencies such as the FDA and EMA impose stringent standards for clinical trial design, data collection, and drug approval, which can prolong the time and increase the cost of bringing DNA repair drugs to market.
Opportunities
Targeted combination therapies exploiting synthetic lethality
One highly anticipated opportunity in the DNA repair drugs market is the development of targeted combination therapies exploiting synthetic lethality. Synthetic lethality occurs when the simultaneous disruption of two specific genes or pathways leads to cell death, while disruption of either gene alone is tolerated. Exploiting synthetic lethality offers a promising approach for selectively targeting cancer cells with defects in DNA repair pathways, such as those with BRCA mutations.
By combining DNA repair inhibitors with other targeted agents or immunotherapies, researchers aim to enhance treatment efficacy and overcome resistance mechanisms, potentially leading to improved outcomes for patients with various types of cancer.
- In January 2023, AstraZeneca announced the potential launch of gene therapy for severe hemophilia A, positioning it as the frontrunner in the U.S. DNA repair drugs market. This innovative therapy aims to mitigate bleeding events, reduce reliance on replacement factor VIII (FVIII), and improve overall outcomes for individuals with severe hemophilia A.
Gene editing technologies for precision DNA repair
Another eagerly awaited opportunity lies in the advancement of gene editing technologies for precision DNA repair. Techniques such as CRISPR-Cas9 offer unprecedented capabilities for precisely modifying DNA sequences, including repairing disease-causing mutations and restoring normal function to damaged genes. By harnessing these technologies, researchers envision the development of novel therapies for genetic disorders characterized by impaired DNA repair mechanisms.
In the DNA repair drugs market, gene editing holds promise for enhancing the effectiveness of these drugs by enabling targeted modifications to specific genomic loci, thereby optimizing treatment outcomes while minimizing off-target effects. As gene editing tools continue to evolve and become more accessible, they represent a transformative opportunity for advancing the field of DNA repair and personalized medicine.
- In December 2023, CRISPR 2.0 heralded a new era of gene editing as it gears up for clinical trials. With the landmark approval of the first CRISPR therapy, the stage is set for treatments leveraging advanced, more efficient, and precise genome editors, promising transformative breakthroughs in healthcare.
- In October 2022, Vertex Pharmaceuticals unveiled Exa-cel (CTX001), a CRISPR/Cas9-based breakthrough targeting transfusion-dependent beta-thalassemia (TDT) and sickle cell disease. This milestone signifies a significant advancement in gene editing technology, promising enhanced treatment options for patients with these debilitating conditions.
Drugs Type Insights
The PARP inhibitors segment held the largest share of the DNA repair drugs market in 2024. Recent advancements in DNA repair drugs have focused on PARP and ATM kinase inhibitors. PARP has been a revolutionary presence in the industries of chronic illnesses such as cancer and Alzheimer's due to its regulatory function in several pathways. PARP inhibitors, such as olaparib and niraparib, have gained prominence in cancer therapy, particularly for treating BRCA-mutated tumors by exploiting synthetic lethality.
The ATM kinase segment is expected to witness the fastest growth during the forecast period. ATM kinase inhibitors, like AZD0156 and M3541, are being investigated for their potential to enhance the efficacy of radiotherapy and chemotherapy by targeting the ATM-mediated DNA damage response pathway. These drugs hold promise for improving treatment outcomes and overcoming resistance mechanisms in various cancers, representing a significant advancement in precision medicine approaches for DNA repair-targeted therapies.
Application Insights
The most recent and notable segments in the DNA repair drugs market are cancer therapy and genetic disorders segments. The cancer therapy segment held the dominating share of the market in 2024. Here, DNA repair drugs target specific pathways involved in repairing DNA damage, particularly in tumors with defective repair mechanisms, such as those caused by BRCA mutations. These drugs offer promising options for personalized cancer treatment and are continuously being explored in clinical trials for various cancer types.
Besides the cancer therapy segment, the genetic disorders segment is observed to witness a significant rate of expansion during the forecast period. In genetic disorders, DNA repair drugs aim to address underlying defects in DNA repair pathways associated with conditions such as neurodegenerative diseases, immune deficiencies, and rare genetic syndromes, offering potential therapeutic interventions for patients with these challenging conditions.
Distribution Insights
The hospital pharmacies segment held a significant share of the in 2024. These facilities play a crucial role in dispensing DNA repair drugs to patients undergoing cancer treatment or genetic disorders requiring specialized care. These pharmacies are equipped to handle complex medication regimens and provide comprehensive support services, ensuring timely access to DNA repair therapies within the hospital setting.
The specialty clinics segment is projected to experience substantial growth in the coming years. Specialty clinics dedicated to oncology or genetic medicine offer specialized expertise in managing patients with DNA repair deficiencies, facilitating the delivery of personalized treatment strategies and targeted therapies tailored to individual genetic profiles.
Regional Insights
In 2024, North America, particularly the United States, stands out as the most dominant region in the DNA repair drugs market. This prominence is primarily attributed to several factors, including robust healthcare infrastructure, extensive research and development activities, and a high prevalence of cancer and genetic disorders necessitating DNA repair interventions. The United States boasts advanced healthcare facilities and a well-established pharmaceutical industry, facilitating the development, production, and distribution of DNA repair drugs. Moreover, the country is home to numerous renowned research institutions and biotechnology companies actively engaged in pioneering advancements in DNA repair therapeutics.
Canada, another critical market within North America, also contributes to the region's dominance in the DNA repair drugs market. With a strong emphasis on healthcare innovation and a supportive regulatory environment, Canada serves as a vital hub for clinical trials and collaborative research endeavors focused on DNA repair mechanisms and targeted therapies. Mexico further bolsters North America's position in the DNA repair drugs market, benefiting from increasing investments in healthcare infrastructure and expanding access to advanced medical treatments.
Asia Pacific region, particularly in countries such as China, Japan, India, and Australia, is emerging as a significant presence in the DNA repair drugs market. Rapidly growing economies, rising healthcare expenditures, and a large patient population afflicted with cancer and genetic diseases drive the demand for innovative DNA repair therapies in this region. As these countries continue to invest in healthcare infrastructure and research capabilities, they present lucrative opportunities for market expansion and collaboration in the field of DNA repair drugs.
DNA Repair Drugs Market Companies
- Abbvie Inc.
- Amgen Inc.
- Abbott
- Astrazeneca Plc
- Bayer Ag
- Bristol-Myers Squibb Co.
- Eli Lilly And Co.
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences Inc.
- Gsk Plc.
- Johnson & Johnson Services Inc.
- Merck & Co. Inc.
- Merck Kgaa
- Novartis Ag
- Pfizer Inc.
- Sanofi
Recent Developments
- In June 2023, AstraZeneca introduced IMJUDO (tremelimumab) in the UAE, pioneering cancer treatment in the Middle East. The groundbreaking medication heralds a new era in cancer therapy, offering hope to patients and positioning the UAE at the forefront of medical innovation in the region.
- In June 2023, the University of Saskatchewan (USask) initiated a pioneering clinical trial to enhance ovarian cancer treatment, setting a global precedent. This innovative approach not only promises to enhance patients' quality of life but also aims to streamline clinical practices and reduce healthcare costs associated with ovarian cancer management.
- In December 2024, Tasca Therapeutics launched with $52 million in Series A funding to advance its drug discovery platform, including progressing its lead candidate CP-383 (a potential DNA repair-targeting drug for tumors) into phase 1/2 clinical studies.
https://www.businesswire.com - In September 2025, Merck announced FDA approval of Keytruda Qlex (pembrolizumab and berahyaluronidase alfa-pmph) for subcutaneous injection for specific solid tumor indications.
https://www.merck.com
Segment Covered in the Report
By Drug Type
- PARP Inhibitors
- ATM Kinase Inhibitors
- ATR Inhibitors
- DNA-PK Inhibitors
- BER Inhibitors
- NER Inhibitors
- Mismatch Repair (MMR) Inhibitors
- Homologous Recombination (HR) Inhibitors
- Non-Homologous End Joining (NHEJ) Inhibitors
By Application Type
- Cancer Therapy
- Genetic Disorders
- Neurodegenerative Diseases
- Immune Deficiencies
- Rare Genetic Syndromes
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Specialty Clinics
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting