What is Meningococcal Vaccine Market Size?
The global meningococcal vaccine market size is estimated at USD 4.02 billion in 2025 and is predicted to increase from USD 4.28 billion in 2026 to approximately USD 6.63 billion by 2034, expanding at a CAGR of 5.81% from 2025 to 2034.
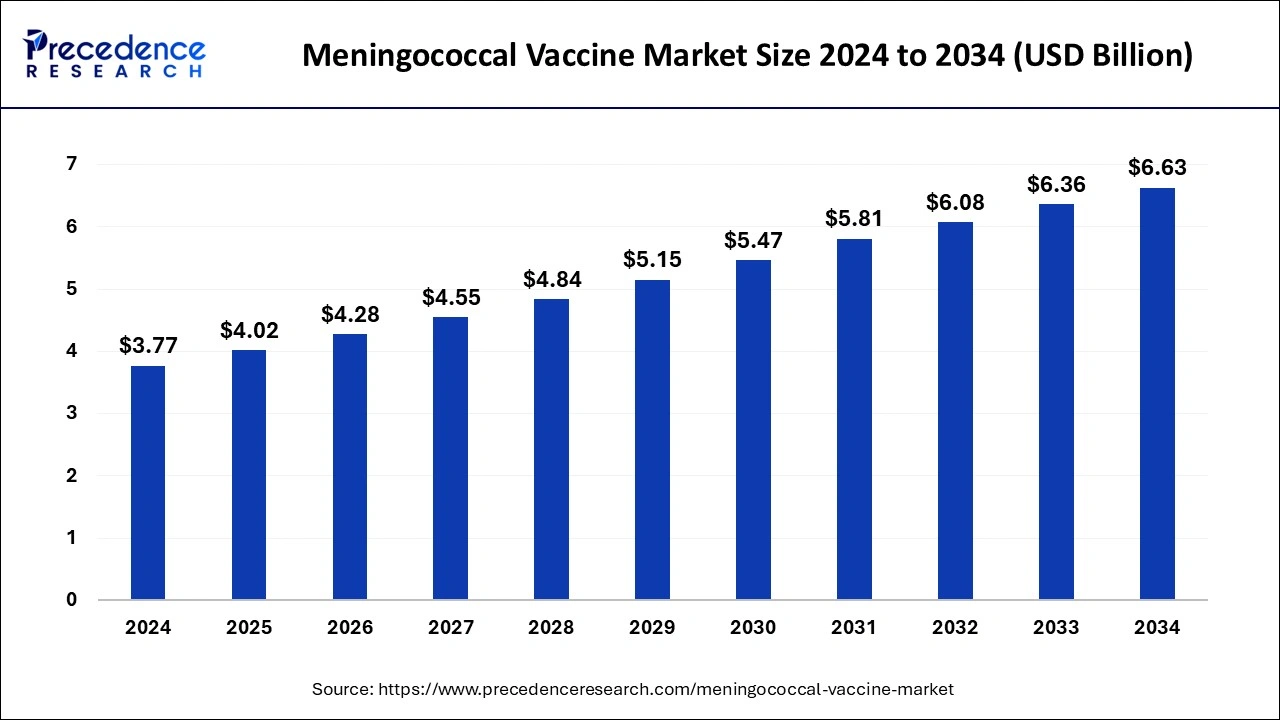
Market Highlights
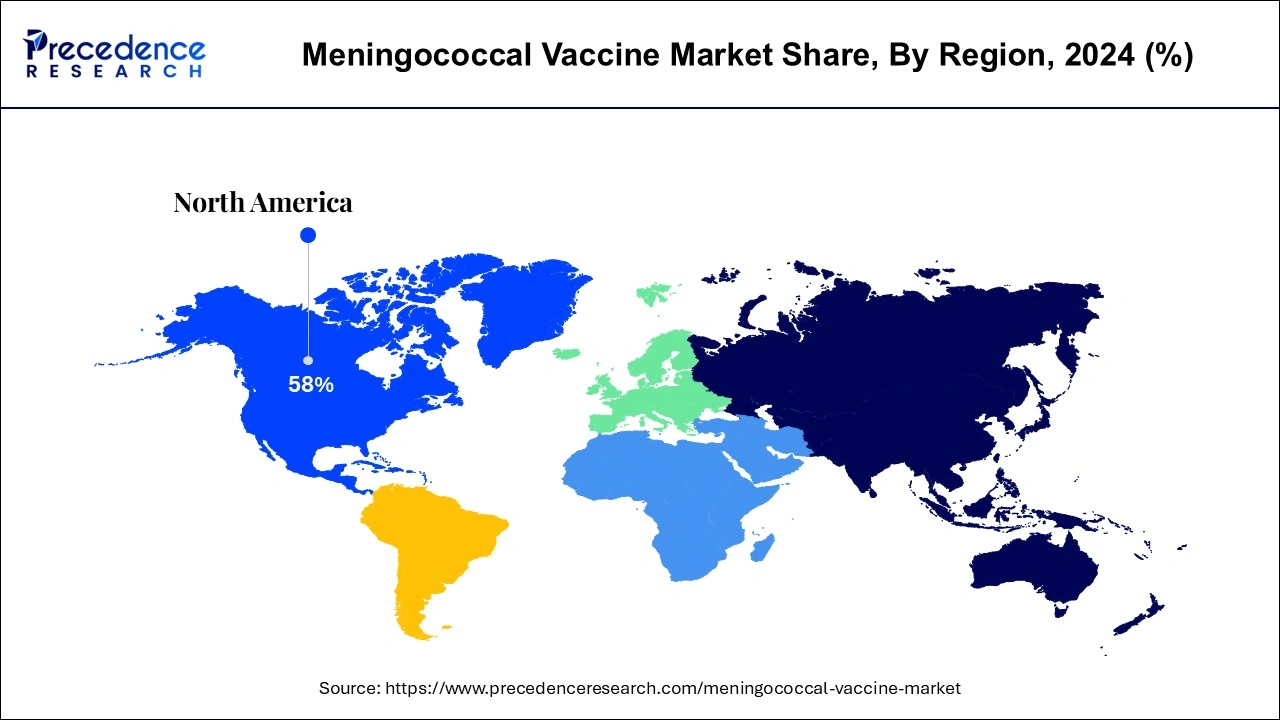
- North America dominated the global meningococcal vaccine with largest market share of 58% in 2024.
- By type, the conjugate vaccines segment accounted for the dominating share of the market in 2024.
- By type, the polysaccharide vaccines segment is expected to witness considerable growth in the global market over the forecast period.
- By end-user, the adult segment held the largest share of the market in 2024.
- By end-user, the children segment is expected to grow significantly in the market during the forecast period.
How is the Meningococcal Vaccine Strengthening?
Meningococcal disease is a rare, life-threatening, and serious disease. This disease is caused by a bacteria known as Neisseria meningitidis. It is an infection of the brain and spinal cord and can cause blood infections, too. Meningococcal vaccines play an effective role in protecting the body against infection from Neisseria meningitidis bacteria that cause meningococcal disease. By vaccination, an individual can fight against meningococcal infection. Meningococcal vaccines help to reduce the prevalence of meningitis outbreaks as well as protect and save valuable lives.
Meningococcal Vaccine Trials Market Growth Factors
- In the meningococcal vaccine market, the rising susceptibility of the vulnerable population group to bacterial attack is a major growth factor.
- State and federal support in the forms of grants and investments helps research and developmental activities in the meningococcal vaccine market.
- Rising awareness about the condition and increasing healthcare expenditure drives the growth of the meningococcal vaccine market.
Meningococcal Vaccine Market Outlook:
- Global Expansion: Specifically, the rising public health initiatives, a shift from older polysaccharide vaccines to novel conjugate vaccines, and the development of newer vaccines to cover more serogroups are propelling the global progression.
- Major Investors: Involvement of giant pharmaceutical companies, like Pfizer Inc., GSK Inc., and other global health firms, is actively investing in advancing R&D activities of vaccines, with expanded production.
- Industry Overview: Especially, the market is characterised by robust competition between significant companies and an emphasis on technological breakthroughs, like evolving more efficient quadrivalent and conjugate vaccines.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 4.02 Billion |
| Market Size in 2026 | USD 4.28 Billion |
| Market Size by 2034 | USD 6.63 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 5.81% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, End-user, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing prevalence of meningitis
The increasing prevalence of meningitis across the globe is expected to boost the growth of the meningococcal vaccine market. Meningococcal disease is a serious health condition that requires immediate treatment as it affects the thin layers of tissue that cover and protect the brain and spinal cord. The proper vaccination against meningococcal infection helps prevent meningococcal disease, which is caused by Neisseria meningitidis bacterium.
Vaccines target specific serogroups of meningococcal bacteria, such as serogroups A, B, C, W, and Y, which cause meningococcal disease. Meningococcal vaccines are widely used to immunize children and infants against Neisseria meningitides bacteria. Therefore, increasing cases of meningococcal disease are expected to spur the demand for meningococcal vaccine market during the forecast period.
Restraint
High cost
The high cost associated with meningococcal vaccines is projected to hamper the market's growth. It can be a financial burden for an individual to purchase this vaccine. High costs may discourage poor people from seeking vaccinations. In addition, the lack of adequate healthcare infrastructure in lower-income countries may limit the adoption and restrict the expansion of the global meningococcal vaccine market during the forecast period.
Opportunity
Rising awareness programs
The increasing awareness programs regarding timely vaccination to fight against meningococcal disease, coupled with supportive government and NGO incentives, are projected to create a significant growth opportunity for the meningococcal vaccine market. Several campaigns and awareness programs, such as the Meningitis Research Foundation of Canada, the National Meningitis Association (NMA), the Meningitis B Action Project, and other awareness programs. Such awareness programs assist in fueling continuous research and innovative approaches for the prevention of meningococcal disease. Thereby driving the expansion of the meningococcal vaccine market.
Segment Insights
Type Insights
The conjugate vaccines segment accounted for the dominating share of the meningococcal vaccine market in 2024 owing to its high efficacy and immunization to fight against meningococcal disease. Conjugate meningococcal vaccine protects against meningococcal group A and C disease. These vaccines are recommended by healthcare professionals, particularly for infants and adolescents.
The polysaccharide vaccines segment is expected to witness considerable growth in the global meningococcal vaccine market over the forecast period. Meningococcal polysaccharide vaccine protects from bacterial meningitis. This vaccine is recommended by healthcare professionals for certain risk groups to control the meningococcal epidemic, and it is available in different forms, such as bivalent, trivalent, and tetravalent. Thereby driving the segment's growth.
End-user Insights
The adult segment held the largest share of the meningococcal vaccine market in 2024. The growth of the segment is driven by increasing cases of meningococcal disease in adults. Adults who travel to areas with a high incidence of the disease are at higher risk of contracting the disease.
The children segment is expected to grow significantly in the meningococcal vaccine market during the forecast period owing to the rising awareness campaign by the government and NGOs about meningococcal disease coupled with increasing emphasis on vaccination as an effective and preventive measure. Routine vaccination is recommended for infants with meningococcal disease. Additionally, the ongoing clinical trials to develop novel vaccines for children are expected to fuel the segment's expansion.
Regional Insights
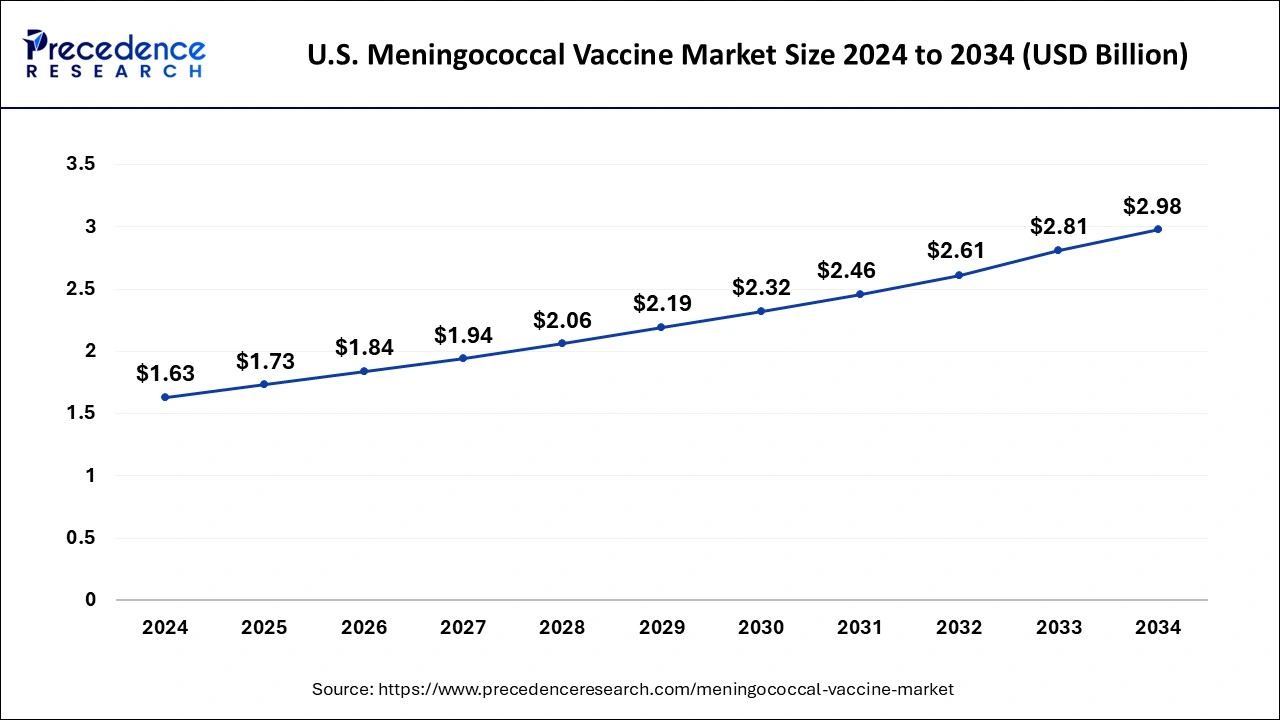
U.S. Meningococcal Vaccine Market Size and Growth 2025 to 2034
The U.S. meningococcal vaccine market size is exhibited at USD 1.73 billion in 2025 and is projected to be worth around USD 2.98 billion by 2034, poised to grow at a CAGR of 6.22% from 2025 to 2034.
How did North America Dominate the Market in 2024?
North America dominated the global meningococcal vaccine market in 2024. The region's growth is driven by the presence of sophisticated healthcare infrastructure, increasing prevalence of meningococcal diseases, access to effective treatment, and rise in government awareness campaigns to educate regarding the risks involved in meningitis and its required preventive measures. Moreover, supportive government policies and rising research activities focused on vaccine development are some of the prime factors expected to drive the growth of the North American market.
Among all countries, the United States is the largest meningococcal vaccine market owing to the well-developed healthcare infrastructure, rise in healthcare expenditure, presence of major pharmaceutical companies, rise in the incidence of meningitis, and favorable government initiatives for disease prevention. As a result, the demand for meningococcal vaccines has significantly increased, and timely vaccination is being carried out in the United States. Furthermore, prominent market players are investing highly in the development of an effective meningococcal vaccine in the region.
The incidence of meningococcal disease is rising in the United States. According to the CDC report in April 2024, as of March 25, 143 cases of meningococcal disease have been reported in the United States, compared with 81 for the same period in 2023. For all of 2023, 422 cases were reported, the highest annual figure since 2014.
- In March 2024, The American Society for Meningitis Prevention (ASMP) launched to empower Americans to take action to prevent meningococcal meningitis, a life-threatening bacterial infection. Founded by Patti Wukovits and Alicia Stillman, two mothers who tragically lost their daughters to meningococcal meningitis, ASMP aims to raise awareness among adolescents, young adults, parents, healthcare providers, and policymakers about meningococcal meningitis and the critical role of comprehensive vaccination in preventing it.
Expanding Awareness Regarding Meningococcal Vaccines is Impacting Europe
Europe is anticipated to grow at a notable growth rate in the meningococcal vaccine market during the forecast period. The fastest growth of the region is attributed to the increasing cases of meningitis, improving access to healthcare systems, supportive government initiatives to combat the rising prevalence of the disease, and increasing new research and development activities. Additionally, rising awareness of meningococcal disease and the significance of timely vaccination are expected to contribute to the growth of the region. Partnership and collaboration among key market players in the region are expected to propel the growth of the meningococcal vaccine market.
- In July 2022, Abacus Diagnostics announced the launch of a new rapid PCR test in the European markets for the fast identification of viruses causing viral meningitis and encephalitis. Neisseria meningitidis, or meningococcus, is a bacterium that can cause meningitis and other forms of meningococcal disease.
- In May 2024, according to the European Centre for Disease Prevention and Control, ECDC is monitoring reports from 3 countries (France, the United Kingdom, and the United States) of cases of invasive meningococcal disease (IMD) associated with travel to the Kingdom of Saudi Arabia (KSA).
Major Discussion on MenACWY for Adolescents: Encourages the German Market
A notable growth in Germany is fueled by the recent significant discussion and modeling studies (published in 2024 and 2025), which have estimated the potential public health impact and affordability of unveiling routine quadrivalent MenACWY (serogroups A, C, W, and Y) vaccination for adolescents.
Persistent Vaccine Launches & Approvals are Promoting the Asia Pacific
Ongoing expansion in the meningococcal vaccine market of ASAP is comprehensively transformed by a rise in vaccine introductions and their approvals, such as in July 2025, the Vietnam Vaccine Joint Stock Company (VNVC) started administering the MenACWY vaccine from Sanofi, putting efforts to accelerate access to quadrivalent vaccines in the region. Besides this, in August 2024, China's Center for Drug Standard Control Organization (CDSCO) approved a Phase III clinical trial for a Meningococcal (A, C, Y, W, X) polysaccharide conjugate vaccine, shifting towards local development of wider coverage vaccines.
Expanded Surveillance and Policy Review: Bolster the Chinese Market
China is experiencing a growing requirement for improving national surveillance to change serogroup distribution and carriage rates. Such as the current vaccination policy is under review, considering the introduction of more conjugate and potentially MenB vaccines into the national immunization program (EPI) for enhancing affordability and public health outcomes.
Meningococcal Vaccine Market: Value Chain Analysis
- R&D
It mainly emphasises the widespread coverage, combination vaccines (pentavalent MenABCWY), boosting accessibility, and use of advanced technologies, such as reverse vaccinology and nanotechnology.
Key Players: Merck & Co., Inc., Sanofi, Serum Institute of India Pvt. Ltd., etc. - Formulation and Final Dosage Preparation
Vaccines are specifically formulated as conjugate vaccines by linkage of the bacterial polysaccharides to a carrier protein, and further reconstituted as a lyophilised (freeze-dried) component with a liquid diluent immediately before intramuscular administration.
Key Players: GSK Inc., Sanofi, Serum Institute of India Pvt. Ltd., etc. - Patient Support & Services
The firms are exploring healthcare provider consultations, access to vaccination programs, and post-vaccination side effect management.
Key Players: Vaccination Centers & Clinics, INVC, etc.
Top Companies and Their Contributions and Offerings in the Market
- Pfizer Inc. is a leading market player, known for vaccines like Trumenba (MenB) and the recent pentavalent Penbraya (MenABCWY) approved in 2023. They focus on comprehensive serogroup coverage and strong distribution networks.
- Novartis AG was a key player, notably with the MenB vaccine Bexsero and the MenACWY vaccine Menveo. Its vaccines business was acquired by CSL Limited (forming Seqirus) and GlaxoSmithKline.
- Bharat Biotech International Ltd. contributes to global access, identified as a key player in the market, often focusing on affordable vaccines for regions like the Asia-Pacific through partnerships.
- Janssen Pharmaceuticals, Inc. (Johnson & Johnson) is a major pharmaceutical entity, but specific approved meningococcal vaccines were not prominently detailed in the search results, as GSK, Pfizer, and Sanofi generally lead this specific market.
- AstraZeneca plc focuses on a broader Vaccines & Immune Therapies portfolio but is not a primary producer of currently available commercial meningococcal vaccines.
Recent Developments
- In April 2024, Nigeria announced the launch of the new Men5CV vaccine, which offers protection against five strains of the Neisseria meningitidis bacteria (meningococcus) responsible for meningitis. Nigeria becomes the first country globally to launch the vaccine, which is recommended by the World Health Organization (WHO).
- In October 2023, Pfizer Inc. announced that the U.S. FDA had approved PENBRAYA (meningococcal groups A, B, C, W, and Y vaccine), the first and only pentavalent vaccine that provides coverage against the most common serogroups causing meningococcal disease in adolescents and young adults 10 through 25 years of age.
- In July 2023, the Serum Institute of India's (SII) multivalent meningococcal meningitis vaccine received World Health Organisation (WHO) prequalification. MenFive is the first conjugate vaccine to protect against the five predominant causes of meningococcal meningitis in Africa.
Segments Covered in the Report
By Type
- Polysaccharide Vaccines
- Conjugate Vaccines
- Combination Vaccines
- Other Types
By End-user
- Children
- Adults
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting