Emulate Launches Chip-R1 Rigid Chip to Enhance Drug Modeling and ADMET Applications
Emulate, Inc., a pioneer in the cutting-edge in-vitro Organ-Chip models in the world, has introduced Chip-R1™ Rigid Chip, with the intention of reducing drug uptake and improving biological modeling for drug absorption, distribution, metabolism, elimination and toxicity (ADMET) Applications. It is made of low-drug-adsorptive polymers and is further enhanced by the main microfluidic backbone of the organ-on-a-chip, predicting human dosing, even to greater extent for researchers.
The redesign of the vascular channel also makes it possible to achieve physiologically relevant values of shear stress, which is vital in any vascular biology related priming or recruitment of immune cells. On top of that, this model has a thinner and more porous membrane for intercellular crosstalk, which also provides closer similarity to the actual physiological condition.
Chip-R1’s low absorption characteristics make it ideal for pharmacokinetics and pharmacodynamics applications in drug development, where drug exposure is maintained constant and predictable. It keeps most of the microfluidic structure of the Chip-S1® Stretchable Chip, such as the co-culture for the tissue-tissue interface, the separate apical and basal channel curve perfusion, and the Emulate’s equipment compatibility. Chip-R1, with a new polycarbonate tissue culture membrane, is better designed for organ modeling that does not have to be mechanically stretched to achieve physiological activities like peristalsis, such as the kidneys and livers.
Chip-R1 is the latest addition to Emulate’s advanced consumables range and demonstrates the progress made in tackling detection of hepatotoxicity as compared to Chip-S1, making the use of Organ-Chips for scientists who could not previously use them due to concerns over drug absorption effects on the accuracy of assessing toxicity of small molecule lipophilic drug candidates.
Latest Announcements by Company Leaders for In-vitro Toxicology Testings
- Erik Fyrwald, CEO of Syngenta, recently spoke about the increasing adoption of in-vitro toxicology testing in the agricultural sector. Fyrwald explained that Syngenta is investing in developing more advanced in-vitro testing methods for pesticide safety, to comply with global regulatory standards and ensure environmental protection.
- James C. Foster, CEO of Charles River Laboratories, recently stated that the in-vitro toxicology testing market is poised for substantial growth, with increasing demand for regulatory-compliant alternatives to animal testing. He highlighted Charles River's continuous investment in expanding its in-vitro testing services, such as human cell-based assays and organ-on-a-chip technologies.
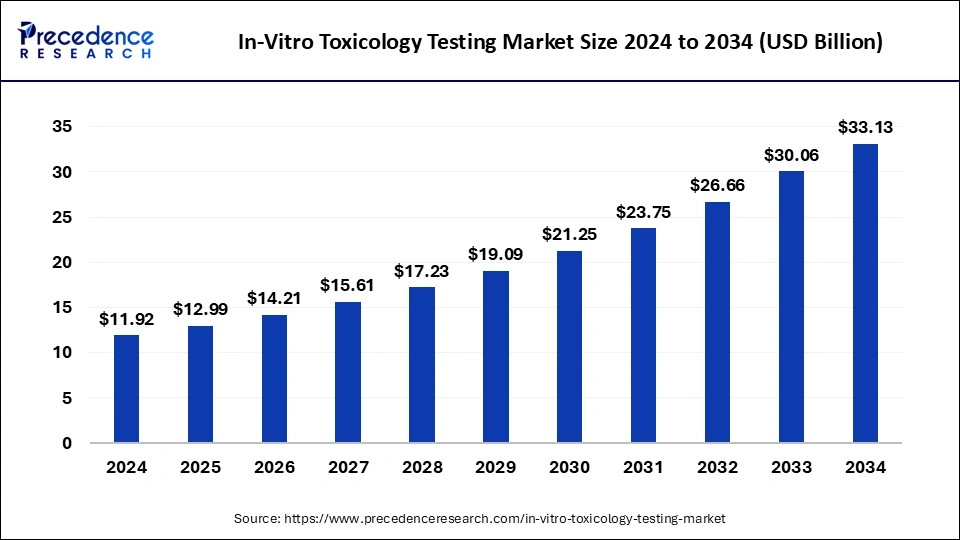
In-Vitro Toxicology Testing Market Size and Growth 2024 to 2033
The global in-vitro toxicology testing market size was valued at USD 11.92 billion in 2024 and is expected to reach around USD 30.06 billion by 2033, at a CAGR of 10.82% from 2024 to 2033. The North America in-vitro toxicology testing market size surpassed USD 5.18 billion in 2023.
In-Vitro Toxicology Testing Market Leading Players
- Charles River Laboratories International, Inc.
- SGS S.A.
- Merck KGaA
- Eurofins Scientific
- Abbott Laboratories
- Laboratory Corporation of America Holdings
- Evotec S.E.
- Thermo Fisher Scientific, Inc.
- Quest Diagnostics Incorporated
- Agilent Technolgies, Inc.
- Catalent, Inc.
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- BioIVT
- Gentronix
- Labcorp Drug Development
- Acea Biosciences Inc.
- Cyprotex Plc
- Ge Healthcare
- Perkinelmer Inc.
- Qiagen Nv
- Biognosys Ag
- Imquest Biosciences Inc.
- Lonza Group Ltd.
- Promega Corporation
- Stemina Biomarker Discovery Inc.
- Vistagen Therapeutics Inc.
- Xenometrix Ag
- MB Research Laboratories
Recent Potential for the In-vitro Toxicology Market
- Heightened governmental interventions and organizational measures to eliminate the use of test animals.
- Expanded resources for in vitro test methods to save animals, humans, and the environment.
- Increasing need for inexpensive and safer substitutes for animal testing in the cosmetic, dietary, and pharmaceutical industries.
- Emerging trends in the healthcare field, especially the development of 3D cell culture and high throughput technology.
- Multiple systems positioned for correct data analysis and efficient strategies have been automated.
- More advanced technologies that will provide more accurate information concerning the toxicity of various chemical substances without the use of animal subjects.
We’ve prepared a service to support you. please feel free to contact us at sales@precedenceresearch.com | +1 804 441 9344