Cannabis Pharmaceuticals Market Will Grow at CAGR of 61.40% By 2032
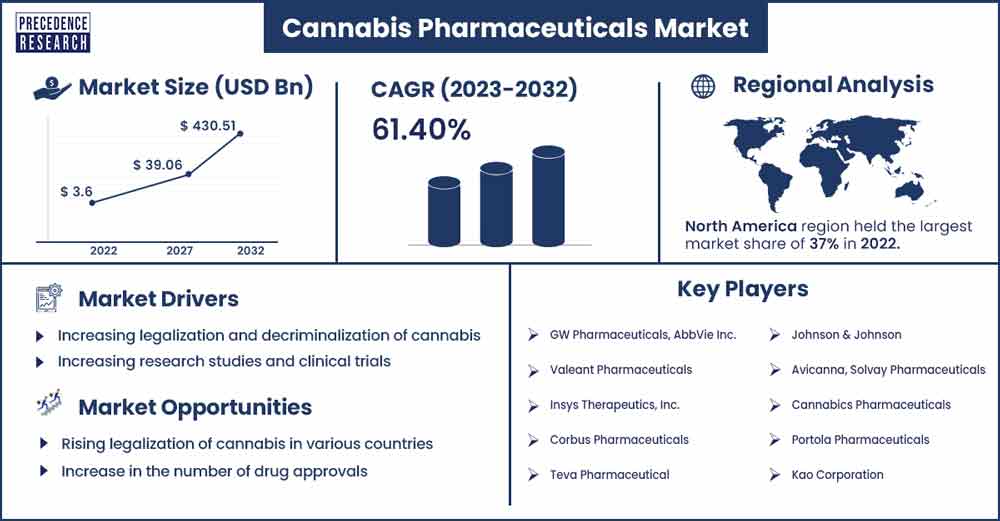
The global cannabis pharmaceuticals market size was exhibited at USD 3.6 billion in 2022 and is anticipated to reach around USD 430.51 billion by 2032, growing at a CAGR of 61.40% from 2023 to 2032.
Market Overview
Cannabis has been utilized as a medicine for several years. Also known as marijuana, weed, or pot, cannabis refers to a group of plants with characteristic psychoactive properties. Cannabis is mainly classified into three subspecies: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. Cannabinoids are found in the Cannabis sativa plant, and they are naturally occurring compounds. Researchers have found that the cannabis plant produces around a hundred cannabinoids and nearly three times that of non-cannabinoid chemicals.
The most commonly known cannabinoids are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is considered the main psychoactive ingredient in cannabis. The introduction of cannabis has revolutionized the pharmaceutical industry. Drugs made from cannabis are widely used for treating several chronic diseases such as arthritis, cancer, and mental disorders like depression, epilepsy, anxiety, migraines, Parkinson’s, and Alzheimer’s disorders. The pharmaceutical company follows stringent safety measures while producing drugs using cannabis.
Growth Factors
The global cannabis pharmaceutical chemicals market is driven by several factors, including supportive government initiatives, rising demand for advanced drugs, rapid growth of the pharmaceutical sector, growing investments in the healthcare sector, increasing awareness among consumers regarding the health benefits of cannabis, expanding use of CBD as an active pharmaceutical ingredient (API) in drug products, rising uses for cannabis in pharmaceutical applications, increasing number of research studies supporting the benefits of cannabis in the treatment of several chronic diseases.
Additionally, the market is growing as a result of the increasing prevalence of chronic diseases such as migraine, arthritis, cancer, epilepsy, tumors, glaucoma, Alzheimer’s, schizophrenia, genetic disorders, multiple sclerosis, and other chronic pain conditions. Furthermore, the legalization of cannabis across various countries toward favoring health concerns is expected to fuel the market’s expansion.
Regional Insights
North America is expected to dominate the market during the forecast period. The North American cannabis-based pharmaceuticals landscape has experienced substantial market growth in recent years, driven by several factors such as an increasing number of biopharmaceutical and pharmaceutical companies, rising awareness regarding the benefits of medical cannabis, sophisticated healthcare infrastructure, novel product development, growing need for life-saving treatment, emerging applications of pharmaceutical cannabis, supportive government initiatives. The developed countries in the region, such as the United States and Canada, are the largest contributors to the market.
Due to the increasing investment in the development of new pharmaceutical drugs, increasing investment in R&D activities, growing demand for personalized medications, rising advancement in drug discovery and development, growing demand for cannabis-based pharmaceuticals, and increasing incidence of chronic diseases such as cancer, depression, arthritis, diabetes, glaucoma, sleep apnea, migraines, chronic pain, epilepsy, acquired immunodeficiency syndrome (AIDS), genetic disorders, Alzheimer’s, Multiple Sclerosis, and others.
For instance, according to an external source, six in every ten individuals in the US have a chronic disease, and four in ten in the US have two or more chronic diseases. The United States and Canada have all legalized the use of cannabis. Furthermore, the legalization in developed countries is expected to spur the demand for cannabis pharmaceuticals and drive revenue growth of the market in the region.
- In April 2023, the National Conference of State Legislature allowed the medical use of cannabis-based products in 38 states, three territories, and the Columbia District. Thus, this is expected to propel the cannabis pharmaceuticals market growth in the region during the forecast period.
Cannabis Pharmaceuticals Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 5.79 Billion |
| Projected Forecast Revenue by 2032 | USD 430.51 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 61.40% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing research studies and clinical trials
The increasing number of research studies and clinical trials supporting the benefits of cannabis in the treatment of several chronic disorders is expected to fuel the market growth during the forecast period. In June 2022, Zynerba Pharmaceuticals Inc., a leader in innovative pharmaceutically-produced transdermal cannabinoid therapies, presented an oral podium presentation and a poster presentation at the American Society of Clinical Psychopharmacology Annual Meeting (ASCP 2022) that was held from May 31 – June 3, 2022.
The presentation titled “Long-Term Safety and Sustained Efficacy of ZYN002 Cannabidiol Transdermal Gel in Children and Adolescents with Fragile X Syndrome (ZYN2-CL-017)” includes data demonstrating that the ongoing long-term safety and efficacy trial of Zygel in children and adolescents with FXS, improvement was seen in Social Avoidance in the total population, with the most significant progress in patients with complete methylation of their FMR1 gene.
Increasing demand for cannabis in the pharmaceutical sector
Pharmaceuticals made from cannabis are widely used to treat a variety of illnesses, such as cancer, migraine, arthritis, Alzheimer’s, sleep apnea, multiple sclerosis, epilepsy, AIDS, depression, glaucoma, and various neurological disorders. Cannabis is a psychoactive drug derived from the cannabis plant. THC and CBD are the two major cannabinoids extracted from this plant, providing medical benefits. Cannabis is an herbal and psychoactive drug that is derived from the cannabis plant. The FDA-approved cannabis-related drug products such as Epidiolex, Marinol, Sativex, Syndros, and Cesamet are rapidly gaining attention.
Restraint
Adverse effects
During the projected period, the Cannabis Pharmaceuticals market’s expansion is anticipated to be constrained by the high risk associated with the overdose of cannabis consumption, such as severe mental and heart issues. There are some short-term effects of cannabis use, such as impairing the ability to think temporarily, ability to harm your mental health, and sometimes making it harder to remember things. Cannabis, in the long term, might harm your lungs and create breathing problems. Cannabis usage is associated with a higher chance of developing anxiety and depressive disorders.
Opportunities
Rising legalization of cannabis in various countries
The increase in the legalization of cannabis in various countries is expected to boost the growth of the mouth ulcer treatment market. Legalization of cannabis for medical use in various countries such as the U.S., Australia, Thailand, Canada, Argentina, Australia, Chile, Cyprus, Germany, Colombia, Croatia, Greece, Israel, Italy, Jamaica, Lithuania, North Macedonia, Norway, the Netherlands, Luxembourg, and South Korea has spurred research and development activities to develop cannabis-based drugs. The rising acceptance of cannabis in various developing and developed countries for medical purposes as a legitimate industry act is a prime factor boosting the revenue growth of the market.
Increase in the number of drug approvals
The increase in the number of drug approvals is projected to offer a lucrative opportunity for Cannabis Pharmaceuticals market growth in the coming years. For instance, In January 2022, Emerald Health Pharmaceuticals Inc. received clearance for its Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) to begin enrolling patients in its Phase 2a clinical trial of EHP-101 for certain relapsing forms of multiple sclerosis (RMS), specifically relapsing-remitting M.S. (RRMS) and active secondary progressive M.S. (SPMS). Such an increase in the number of drug approvals is projected to witness the fastest growth during the forecast period.
Recent Developments
- In April 2023, BAT collaborated with CBD firm Charlotte's Web to develop a drug for an undisclosed neurological condition—a joint venture between BAT's subsidiary AJNA BioSciences PBC and Charlotte's Web. BAT invested USD 10 million in the deal last year, and AJNA each owns 40% of the entity, while BAT controls the remaining stake.
- In April 2023, Hyderabad-based Dr. Reddy's Laboratories and MediCane Health Inc. announced the launch of their therapeutic cannabis product in Germany. Under a collaboration between Dr. Reddy's and MediCane that began in 2021, MediCane will supply medical cannabis products to Dr. Reddy's from its EU GMP-certified facilities based in Portugal while providing logistical and regulatory support.
- In February 2023, BOHECO introduced India's first line of clinically trialled medical cannabis products. Bombay Hemp Company (BOHECO) marked ten years of providing research-led and innovation-backed hemp-based wellness to consumers. On the occasion of its 10th year since inception, the brand reaffirmed its commitment towards patients with the launch of a range of next-generation, clinically trialled cannabis leaf-based products targeted towards various chronic pain and mental health conditions.
- In April 2023, Aurora Cannabis Inc., the Canadian company opening the world to cannabis, and MedReleaf Australia announced the launch of IndiMed Tempo 26, a range of new higher THC dried cannabis products for qualified patients under the MedReleaf Concession Scheme (MCS). Tempo 26 will add to the portfolio of products available, giving doctors the ability to prescribe from a wider range of options for medical cannabis patients.
- In June 2023, Philip Morris International announced its intention to buy the Israeli cannabis tech firm Syqe Medical in a deal worth up to USD 650 million.
Cannabis Pharmaceuticals Market Players
- GW Pharmaceuticals, AbbVie Inc.
- Valeant Pharmaceuticals
- Insys Therapeutics, Inc.
- Corbus Pharmaceuticals
- Teva Pharmaceutical
- Johnson & Johnson
- Avicanna, Solvay Pharmaceuticals
- Cannabics Pharmaceuticals
- Portola Pharmaceuticals
- Kao Corporation
- Ogeda S.A.
- Pfizer
- Bristol-Myers Squibb
Segments Covered in the Report
By Product
- Epidiolex
- Marinol
- Cesamet
- Sativex
By Distribution Channel
- Hospitals
- Online pharmacies
- Retail
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3371
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 9197 992 333