Clinical Laboratory Services Market to Exceed USD 300.54 Bn By 2032
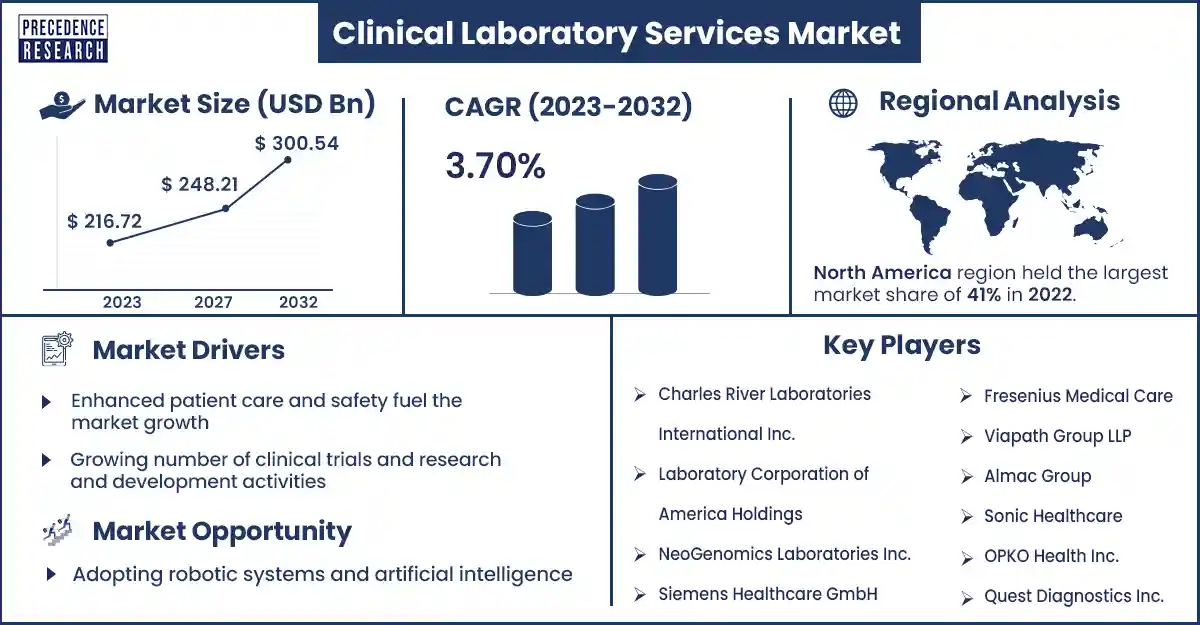
The global clinical laboratory services market size was evaluated at USD 216.72 billion in 2023 and is expected to attain around USD 300.54 billion by 2032, growing at a CAGR of 3.70% from 2023 to 2032. The clinical laboratory services market growth is due to factors such as increasing demand for early diagnostic tests and the increasing burden of chronic diseases.
Market Overview
The clinical laboratory services market provides a wide range of laboratory services that help clinicians diagnose, manage, and treat patients. The market growth deals with factors such as increasing incidence of chronic diseases, growing geriatric population, and also lifestyle-related diseases. During the forecast period, rapid developments in sample preparation and data management due to increasing volumes of testing samples are expected to drive market growth.
Moreover, the increasing rate of chronic diseases, especially among the aging population, is enhancing the demand for clinical laboratory services. In addition, the growing emphasis and awareness of preventive healthcare have increased the demand for laboratory services and stimulated routine health check-ups.
Enhanced Patient Care and Safety Fuel the Market Growth
Clinical laboratories contribute to enhanced safety and patient care in various ways. Firstly, the reduction in fluctuating time for diagnostics tests is critical in emergencies. Appropriate diagnosis of conditions such as sepsis or myocardial infarction can suddenly affect patient survival rates. In clinical laboratory services, medical teams can treat emergency cases on a priority level and expedite the testing procedure, leading to immediate intervention and faster diagnosis, which may drive the clinical laboratory services market.
In addition, clinical laboratories also improve the coordination among healthcare professionals. Laboratory staff can directly communicate with specialists, nurses, and doctors to discuss and collaborate on treatment plans and clarify any uncertainties and test results. This absolute coordination ensures that patient care is coordinated, errors are avoided, and potential delays are eliminated. These major factors can boost market growth.
However, recruitment and staff utilization can restrain clinical laboratory services growth. In many clinical laboratories, an expected amount of time is wasted maintaining manual monitoring procedures, such as the weekly data recording of fridge temperatures. Laboratory staff can spend more time on productive work that adds value to laboratory services supplied to consumers. This situation is complicated by the issues that many laboratories have in retaining and recruiting skilled staff.
Clinical Laboratory Services Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 216.72 Billion |
| Projected Forecast Revenue by 2032 | USD 300.54 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 3.70% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Top Companies in Clinical Laboratory Services Market
- OPKO Health Inc.
- Redcliff Labs
- Sonic Healthcare
- SPT Labtech
- Almac Group
- Viapath Group LLP
- Fresenius Medical Care
- Quest Diagnostics Inc.
- Siemens Healthcare GmbH
- NeoGenomics Labarotories Inc.
- Laboratory Corporation of America Holdings
- Charles River Laboratories International Inc.
Recent Development by Redcliff Labs
- In August 2023, Redcliffe Labs launched Clinical Decision Support in collaboration with Abbott India. This clinical laboratory is powered by innovative technologies such as Artificial Intelligence and Big Data Engines. This modern development aims to revolutionize healthcare by supplying potential solutions to improve patient facility and care.
Recent Development by SPT Labtech
- In November 2023, in America, SPT Labtech launched various unique developments related to its platform named Firefly. This innovation is an all-in-one genomics liquid handling platform. Focused on addressing the bottlenecks of preparation in Laboratory Developed Tests.
Regional Insights
Asia Pacific is estimated to grow fastest in the forecast period. In this region, development and ongoing research activities combined with government initiatives are developing new opportunities for the growth of the clinical laboratory services market. Asia Pacific also has countries like China and India, which are the two countries with the largest population. The population increases the use of clinical laboratories.
In India, clinical laboratory services play an important role in healthcare. Indian Confederation of Medical Laboratory is a national registered Confederation of more than 50 thousand scientists and clinical laboratory technologists working under several private health care and Indian governments across the nation. India, being a developing country, has various health-related issues that clinical laboratories can address.
- In March 2024, Clinisys Laboratory Solutions announced their plans to grow India's workforce by 50% in the year 2024. This includes recruiting 200 more employees. Clinisys Laboratory Solutions is a world supplier of laboratory data systems.
North America dominated the clinical laboratory services market in 2023. In North America, the clinical laboratory service market is being boosted by the increasing prevalence of chronic diseases due to pollution, sedentary lifestyles, and the consumption of junk food. North America is a developed country with a great healthcare infrastructure, and the governments in the region also support and provide necessary financial and other types of help whenever needed. North America has countries like the U.S. and Canada that play a major role in the market growth in the North American region.
In the U.S., clinical laboratories are heavily regulated by the government. In the U.S., each of the clinical diagnostic labs that perform in vitro diagnostic tests has to obey the Centers for Medicaid and Medicare Services and Clinical Laboratory Improvement Amendments, which regulate all laboratories performing testing on people that are calculated to inform the treatment of disease, diagnosis, or prevention. Clinical laboratory improvement amendments require every clinical laboratory to obtain and apply for a certificate that corresponds to the complications of diagnostics tests that the clinical lab performs. Such regulatory bodies are important for maintaining safety standards and proper infrastructure. Apart from this, the U.S. is also prone to various diseases due to unhealthy eating habits, sedentary lifestyle, pollution, and occupational hazards. Obesity is the most common and huge problem in the U.S. According to the CDC, in 2022, 35% of adults from 22 states had obesity.
- In June 2023, OPKO Health Inc. and Pfizer Inc. announced the approval of NGENLA by the FDA. NGENLA is a human growth hormone that should be given once a week for the treatment of pediatric patients of the age 3 group with growth failure issues.
In Canada, Laboratories Canada policy is creating collaborative, innovative, and world-class science research centers across the country. Canada will position itself at the forefront of smart, innovative ways and discoveries of delivering on research priorities for Canadians.
Market Potential and Growth Opportunity
Adopting robotic systems and artificial intelligence
Clinical laboratories enhanced with artificial intelligence are the future. Machine learning software and AI are beginning to combine themselves as tools for accuracy and efficacy within clinical laboratories. In the future, AI and robots used in the clinical laboratory will be self-learning, using inferential procedures, with various ways to enhance the best possible decision, even allowing for missing data. Artificial intelligence programs combined with ambient computing, mixed reality, natural language processing, computer vision, pattern recognition, mathematical modeling, data mining, and statistics will change the way clinical laboratories display and generate clinical information in the future, and robots can reduce human workload and reduce errors. These opportunities may drive the growth of the clinical laboratory services market in the coming years.
Clinical Laboratory Services Market News
In November 2023, in Boston, global healthcare technology Velsera launched new capabilities for its Clinical Genomics Workplace. This innovation is streamlining the path of clinical laboratory professionals to communicate with medical proof and complicated molecular diagnostics reports.
In October 2022, Q2 Solutions launched the first self-collection safety clinical lab panel for United States clinical trial participants by clinical trial laboratory. Q2 Solutions is a leading global clinical trial laboratory technology.
Market Segmentation
By Test Type
- Human and Tumor Genetics
- Clinical Chemistry
- Medical Microbiology and Cytology
- Genetic Testing
- Drug of Abuse Testing
- Other Esoteric Tests
By Service Provider
- Hospital based laboratories
- Standalone laboratories
- Clinic based laboratories
By Application
- Bioanalytical and Lab Chemistry Services
- Toxicology Testing Services
- Cell and Gene Therapy Related Services
- Preclinical and Clinical Trial Related Services
- Drug Discovery and Development Related Services
- Others
Buy this Research Report@ https://www.precedenceresearch.com/checkout/1627
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308