Intravascular Lithotripsy Market Revenue to Attain USD 3.40 Bn by 2033
Intravascular Lithotripsy Market Revenue and Trends 2025 to 2033
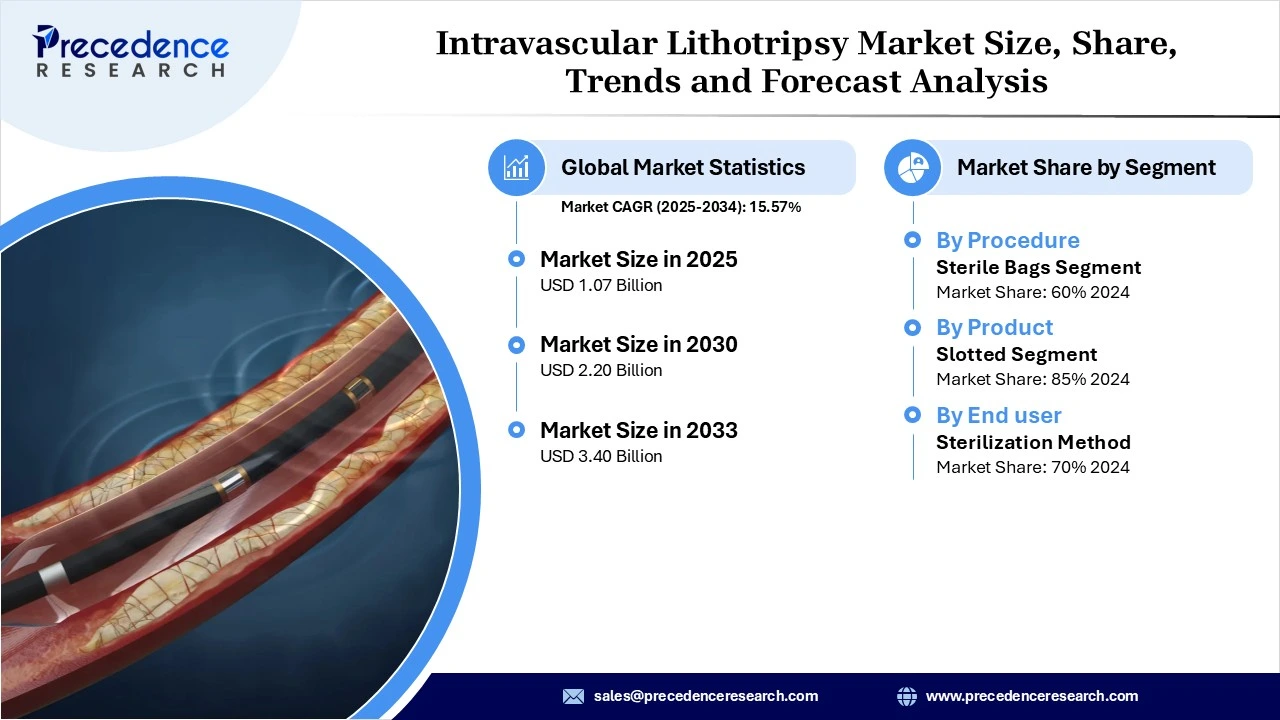
The global intravascular lithotripsy market revenue reached USD 1.07 billion in 2025 and is predicted to attain around USD 3.40 billion by 2033 with a CAGR of 15.57%. This market is expanding due to the rising prevalence of calcified cardiovascular disease, combined with strong clinical evidence, favourable reimbursement updates, and major industry M&A activity, which is accelerating IVL adoption globally.
What is the mechanism of action of intravascular lithotripsy?
The mechanism of action of intravascular lithotripsy (IVL) involves using pulsed sonic pressure waves that specifically disrupt and break down calcium in place, thereby altering the vessel's compliance while minimizing injury and preserving the vessel wall's fibroelastic components. Clinical data showing IVL's safety and effectiveness in heavily calcified lesions, with lower complication rates and improved stent expansion, is quickly increasing clinician confidence and consideration for inclusion in guidelines. The adoption of IVL has been increasing due to the rising number of patients with coronary and peripheral indications. Advances in medical device technology open up new growth prospects.
Segment Insights
- By procedure/application, the coronary artery disease (CAD)segment dominated the market in 2024, as CAD is the most common condition involving calcified arterial plaques that impede blood flow. IVL enables the predictable modification of calcified coronary lesions, thereby enhancing the outcomes of PCI, particularly in complex, heavily calcified anatomies.
- By product/device type, the IVL catheters segment dominated the market with the largest share in 2024. This is because these catheters are the primary devices that deliver the sonic pressure waves directly to calcified plaques, making them essential for the procedure’s effectiveness.
- By end-user/hospital type, the cardiac & cath lab segment dominated the market and is expected to sustain its growth trajectory in the coming years. This is because increasing patient volume in these settings. Their focus on treating coronary artery disease and other vascular conditions ensures high utilization of IVL devices, driving segmental growth.
Regional Insights
North America dominated the intravascular lithotripsy market in 2024 due to early regulatory approvals, established reimbursement pathways, and larger hospital capital budgets. Regulatory approvals and CPT and DRG code progress from the FDA ultimately shorten the time to market entry. In the U.S., there is also a higher volume of PCI procedures performed, along with established interventional treatment teams, which facilitate the rapid adoption of devices and procedures.
Asia Pacific is poised for the fastest growth, driven by the increasing burden of cardiovascular disease, the rise in access to tertiary cardiac centers, and improved access to advanced devices that may increase procedure utilization. Although adoption is lagging behind North America's maturation of regulatory and reimbursement pathways, the engagement of clinicians with local distributors that support the education of newer devices, along with a commitment to China, Japan, and India, is expected to accelerate global uptake and device utilization.
Intravascular Lithotripsy Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 1.07 Billion |
| Market Revenue by 2033 | USD 3.40 Billion |
| CAGR from 2025 to 2033 | 15.57% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Intravascular Lithotripsy Market Key Players
- Shockwave Medical
- Boston Scientific
- Medtronic
- Abbott Laboratories
- Philips Healthcare
- Terumo Corporation
- Becton Dickinson (BD)
- Penumbra, Inc.
- Cook Medical
- Cardinal Health
- Teleflex Incorporated
- Asahi Intecc
- Siemens Healthineers
- Abiomed
- OrbusNeich
- Volcano Corporation
- Avinger Inc.
- Spectranetics (Philips)
- Merit Medical Systems
- C.R. Bard (now BD)
Recent Development
- In March 2025, Shockwave Medical, a part of Johnson & Johnson MedTech, launched the Shockwave Javelin Peripheral IVL Catheter in the U.S., a novel device designed to modify calcium and cross tight vessels in patients with peripheral artery disease, offering safety and efficacy comparable to legacy IVL catheters and strengthening its leading market portfolio.
https://www.jnjmedtech.com
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6882
You can place an order or ask any questions. Please feel free to contact at sales@precedenceresearch.com |+1 804 441 9344