Melanoma Therapeutics Market Will Grow at CAGR of 10.3% By 2032
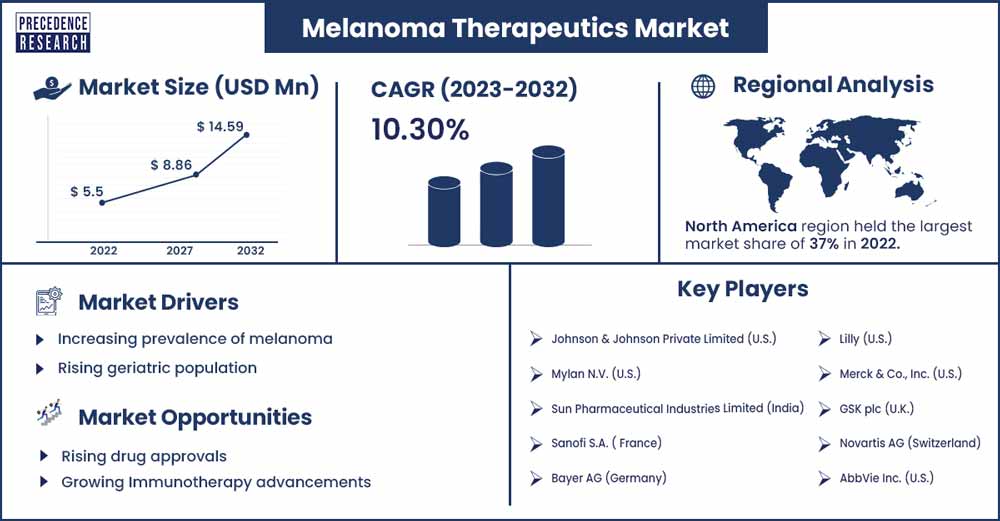
The global melanoma therapeutics market size was exhibited at USD 5.5 billion in 2022 and is anticipated to reach around USD 14.59 billion by 2032, growing at a CAGR of 10.3% from 2023 to 2032.
Market Overview
Melanoma is a type of skin cancer that arises from melanocytes. Melanoma is usually caused by excessive UV light exposure or genetics in some cases. Excessive ultraviolet (UV) exposure from sunlight and tanning beds significantly increases melanoma risk. Melanoma is life-threatening and dangerous as it develops quickly in nearly less than six weeks. It can harm the internal organs very quickly, which makes metastatic melanomas harder to treat.
In the early stage, the treatment of melanomas includes surgical removal of the cancerous tissue. The therapy required if melanoma has spread beyond the skin includes surgery to remove affected lymph nodes, immunotherapy, targeted therapy, radiation therapy, and chemotherapy.
The growth of the global melanoma therapeutics market is driven by several factors, including growth in healthcare infrastructure, funding from both public and private sectors, rise in drug discovery and development, technological advancements, increasing ageing population, growing government-supportive initiatives for early detection and skin cancer treatment, increase in drug approvals, increasing demand for personalized medicine, development of early detection tools, and the robust growth in the production of cost-effective drugs.
In addition, the rising investment in research and development activities and the increase in clinical trials have positively impacted the melanoma therapeutics market. Furthermore, the market is growing as a result of the increasing prevalence of melanoma, which has propelled the demand for advanced melanoma therapies.
- In September 2023, in a multiyear collaboration, Moderna Inc. agreed to pay USD 1.8 billion to a German biotechnology company, Immatics NV to develop cancer therapies using messenger RNA and other technologies.
- In December 2023, an experimental cancer vaccine from Moderna and Merck & Co. continued to show promise in treating melanoma, the evidence shows that combining the shot and immunotherapy Keytruda kept more people alive and disease-free three years after surgery than Keytruda alone.
- In August 2023, Delcath Systems, Inc., announced that the US FDA approved HEPZATO KIT (melphalan/Hepatic Delivery System) as a liver-directed treatment for adult patients with metastatic uveal melanoma (mUM) with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation.
Regional Insights
North America is expected to hold the largest market share over the forecast period owing to the presence of well-established healthcare infrastructure, rising investment in research and development, favorable government initiatives, increasing geriatric population, high burden of skin cancer, rising demand for personalized medicine, increasing awareness for early detection skin cancer treatment, rising strategic initiatives and high healthcare expenditure facilitating in increasing adoption of advanced melanoma treatments.
The presence of major companies operating in the region, including Pfizer Inc., Mylan N.V., Lilly, Merck & Co., Inc., AbbVie Inc., Amgen Inc., Bristol-Myers Squibb Company, AgonOX, Abbott Laboratories, Johnson & Johnson, Bausch Health Companies Inc., and others is expected to fuel the market’s expansion.
The United States is the biggest supporter of the market owing to the significant healthcare expenditures, increasing demand for advanced therapies, an emerging pipeline for skin cancer treatment, ongoing clinical trials, a rise in drug approvals by regulatory bodies, and a rising prevalence of melanoma. With the rapidly rising melanoma cases in the U.S., the market’s growth is expected during the forecast period. According to the American Cancer Society, in the United States, it is also estimated that about 7,990 people are expected to die of melanoma. In addition, the rising approval for drugs by the U.S. Food and Drug Administration that help in the management of melanoma is likely to bolster the market’s growth.
- In October 2023, the FDA approved nivolumab (Opdivo, Bristol-Myers Squibb Company) for the adjuvant treatment of completely resected Stage IIB/C melanoma in patients 12 years and older. Therefore, such recent developments are expected to drive the overall market growth in the region during the forecast period.
Melanoma Therapeutics Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 6.04 Billion |
| Projected Forecast Revenue by 2032 | USD 14.59 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 10.3% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing prevalence of melanoma
The rising cases of melanoma across the globe have led to the increasing adoption of melanoma therapeutics for the treatment of the disease. Melanoma is the most hazardous and aggressive type of skin cancer. Several factors increase the risk of developing melanoma, such as constant exposure to UV radiation, multiple moles, fair skin, a history of sunburns, and genetic factors. According to the data provided by WHO, it is estimated nearly 132,000 cases of skin cancer occur annually. As per the report published by The Skin Cancer Foundation, in recent years, the number of new diagnoses annually rose by 27% in the US. The report also claimed that one in every five US citizens will develop skin cancer by the age of 70. This is anticipated to serve as an opportunity for drug companies to develop effective drugs and therapies to treat melanoma disease.
Rising geriatric population
The surge in the geriatric population is expected to fuel the expansion of the global melanoma therapeutics market in the coming years. As the susceptibility to melanoma increases with age, the average age of diagnosis being around 66, the growing aging population has amplified the demand for melanoma drugs. This, along with advancements in treatment, underscores the potential for market growth.
Restraints
Stringent government regulations
Implementing rigid compliance with regulations is expected to hamper the development of the melanoma therapeutics market. Regulatory authorities of various regions impose different guidelines regarding the approval of melanoma drugs. Over the projected period, the melanoma therapeutics market's expansion is anticipated to be constrained by stringent regulatory framework.
High cost
The high cost associated with the treatment of melanoma is likely to restrain the market’s growth. In addition, lack of awareness and unavailability of appropriate treatment, particularly in underdeveloped countries. In such countries, there are limited healthcare resources unavailable in hospitals and clinics. Therefore, it hinders the market growth.
Opportunity
Rising drug approvals
The rapid rise in the adoption of immunotherapy as an effective treatment for melanoma is projected to boost the global melanoma therapeutics market. Immunotherapy helps to enhance a person's immune system to destroy cancer cells. There are various types of immunotherapies available that can be used for the treatment of melanoma conditions. In addition, the rise in the approval of immunotherapy for melanoma by the Food and Drug Administration is one of the primary growth stimulants for the market.
- In March 2022, The U.S. FDA approved a novel therapy for patients with metastatic or inoperable melanoma, an aggressive type of skin cancer. The treatment developed based on original research conducted at the Johns Hopkins Kimmel Cancer Center is comprised of two immunotherapy agents, relatlimab (anti-LAG-3) and nivolumab (anti-PD-1), which delayed time to cancer progression significantly more than nivolumab alone in a global, multi-center clinical trial. Thereby, driving the market’s growth.
Recent Developments
- In February 2023, Pfizer Inc. announced that the U.S. FDA approved its supplemental New Drug Application (sNDA) for CIBINQO (abrocitinib), expanding its indication to include adolescents with refractory, moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with other systemic drug products, including biologics, or when the use of those therapies is inadvisable.
- In October 2023, 3M young scientists from Bekele developed a soap for treating melanoma, which won him the competition and its grand prize of USD 25,000.
Key Market Players
- Johnson & Johnson Private Limited (U.S.)
- Mylan N.V. (U.S.)
- Sun Pharmaceutical Industries Limited (India)
- Sanofi S.A. ( France)
- Bayer AG (Germany)
- Lilly (U.S.)
- Merck & Co., Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- AbbVie Inc. (U.S.)
- Bausch Health Companies Inc. (Canada)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Amgen Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Novartis AG (Switzerland)
- Genentech, Inc (U.S.)
- AstraZeneca (U.K.)
- DAIICHI SANKYO COMPANY, LIMITED (Japan)
- AB Science (France)
- AgonOX (U.S.)
- Eisai Co., Ltd (Japan)
- Pfizer, Inc. (U.S.)
Market Segmentation
By Product
- Chemotherapy
- Immunotherapy
- Targeted Therapy
- Radiation Therapy
By Drug Type
- Branded Drugs
- Generic Drugs
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3401
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308