Psoriasis Treatment Market Size to Attain USD 57.7 Bn by 2032
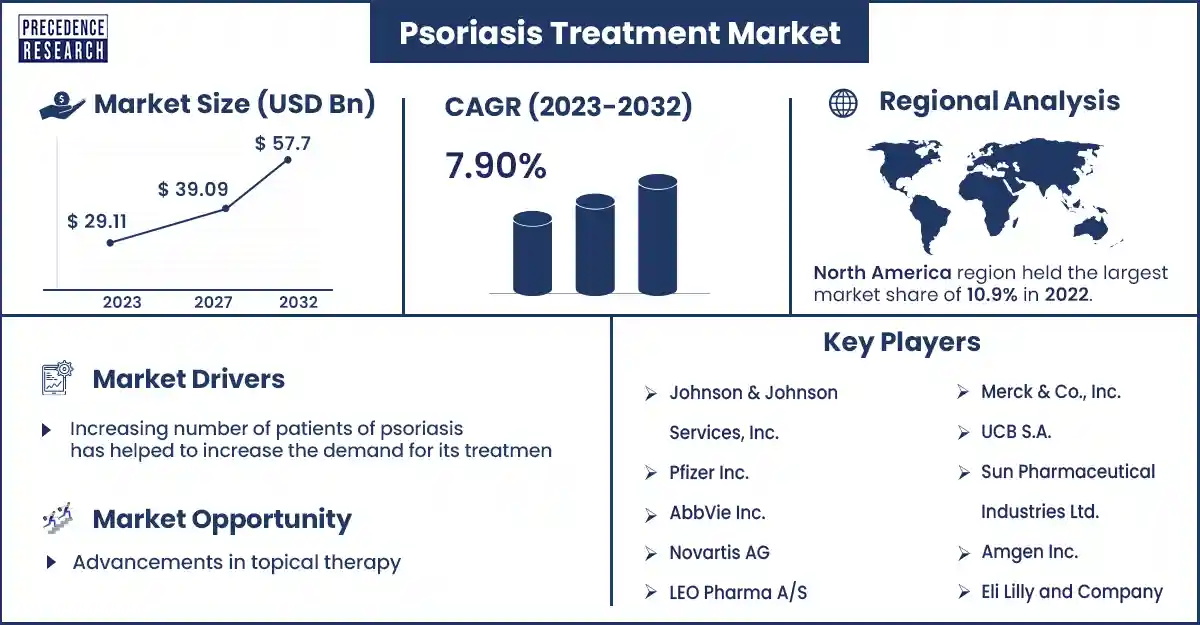
The global psoriasis treatment market size surpassed USD 29.11 billion in 2023 and is estimated to attain around USD 57.7 billion by 2032, growing at a CAGR of 7.9% from 2023 to 2032 The increasing demand for variable frequency drives by several end users drives the VFD market growth. The increasing prevalence of psoriasis over the world is a major factor driving the psoriasis treatment market.
Market Overview
The psoriasis treatment consists of oral medications, light therapy, topical therapy, and creams. The psoriasis treatment market fulfills all the requirements needed to diagnose, treat, and monitor psoriasis disease. Psoriasis is an immunological inflammatory disease resulting from the excessive production of skin cells and arising in a painful and itchy skin condition by plaque formation, red lesions, and inflammation. Psoriasis is classified into two types- Psoriatic Arthritis and plaque psoriasis. Psoriatic arthritis, the increasing prevalence of plaque, and the highly increasing aging population are expected to drive market growth. In addition, the increasing volume of prescribed biological products, favorable refund policies, and a huge pipeline of biosimilar and biologic products are anticipated to enhance the market growth. Moreover, the increasing prevalence of chronic diseases, increasing awareness regarding treatment, increasing collaborations between manufacturers, increasing healthcare spending, and increasing R&D for improvement of treatment are expected to drive the growth of the psoriasis treatments market.
Continuous treatment process fuels the growth of the psoriasis treatment market
Psoriasis disease is a non-curable disease. Once it has occurred or covered the skin areas, then it will not cure. So, continuous treatment is the most suitable process for this disease. Psoriasis can make it hard to concentrate, interfere with sleep, and, most of the time, be very painful. There is presently no permanent treatment for curing psoriasis, but continuous treatment can bring nearly complete suspension of skin patches. Continuous treatment focuses on meeting physical manifestations and minimizing inflammation, including skin lesions. With continuous and effective treatment, a patient living with psoriasis can handle their symptoms properly and aiming remission. Most of the doctors used multiple combinations of treatments to cure psoriasis. Therefore, a continuous treatment process can be the major driver in this market growth.
The market's expansion could be hampered, nevertheless, by the scarcity of available treatment choices. Fewer treatment options are available for psoriasis-affected skin regions. One group of individuals has nail-predominant psoriasis. Moreover, topical creams don't work and don't cover enough ground to justify systemic therapy. Topical medications might result in skin issues, which are typically troublesome in these places, which makes inverse or genital psoriasis an even more serious condition. Those who suffer from psoriasis limited to the scalp may find it difficult to apply topical steroids, or their hair may become shiny. Due to their hair's inability to absorb light, patients with scalp-only psoriasis frequently aren't eligible for appropriate treatment. These are the main causes limiting the market for psoriasis treatments from expanding further.
Psoriasis Treatment Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 29.11 Billion |
| Projected Forecast Revenue by 2032 | USD 57.7 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 7.9% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
The Psoriasis Treatment Market Top Companies
- Evelo Biosciences, Inc
- Lord’s Marke Biotech
- Eli Lilly and Company
- Amgen Inc.
- Sun Pharmaceutical Industries
- UCB S.A.
- Merck & Co., Inc.
- LEO Pharma A/S
- Novartis AG
- AbbVie Inc.
- Pfizer Inc.
- Johnson and Johnson Services, Inc.
Recent Development by Lord’s Mark Biotech
- In November 2023, for the cure and treatment of psoriasis disease, the patented medicine Tinefcon was launched by a subsidiary of Lord’s Marke Industrie, Lord’s Marke Biotech. This product is being launched as a perfect solution for psoriasis in four forms: scalp wash, shower gel, cream, and tablet. The Tinefcon is priced in the range of 799-3900 Rs.
Recent Development by UCB
- In March 2024, to support the leading treatment for the psoriasis community, during the 96th Academy Awards, UCB launched a direct-to-consumer advertising campaign. This advertising was the first direct-to-consumer campaign in the psoriasis disease and dermatology brand.
Regional Insights
North America dominated the psoriasis treatment market in 2023. The increasing prevalence of chronic diseases and psoriasis disease is estimated to drive market growth in North America. The United States and Canada are the major emerging countries in North America.
The increasing prevalence of psoriasis is expected to be 2 to 3 percent of the total population, and roughly millions of patients are found in adults. In the United States, pharmacy claims from geographically diverse and patient-level medical information are systematically updated. The qualified patients in the U.S. aged 18 years initiated a new psoriasis treatment with approval from the U.S. Food and Drug Administration. Considering the cost-effectiveness and clinical evidence of the advantages and harms of psoriasis treatments, associated healthcare costs, and reviewing real-world treatment patterns may help US players. In the United States, people are utilizing reliable data, providing robust and observed treatment patterns for the advanced psoriasis treatment process.
- According to the real-world story, the patients with psoriasis who were using biologics usually lasted over 24 months. These are the major factors responsible for the growth of the psoriasis treatment market in the United States.
- For instance, In October 2023, in the United States, for the treatment of average to severe plaque psoriasis in adults who are candidates for proper phototherapy or therapy, the pharmaceutical company UCB announced the approval of BIMZELX the U.S. Food and Drug Administration.
Market Potential and Growth Opportunity
Advancements in topical therapy
Biological, photodynamic, and systemic therapy are used to treat low-to-high severe types of psoriasis, which account for around 70% of patients receiving topical treatment. In addition to treating a significant portion of psoriasis, topical treatment offers new developments, therapeutic significance, and commercial prospects for the topical administration of steroids for psoriasis. For many patients, the use of biological molecules and novel immunomodulatory delivery systems for topical psoriatic therapy has shown to be a safe and effective choice.
These are the most recent developments in topical medicine. Once the clinical relevance of traditional topicals over psoriasis has been established, their potential to revolutionize and penetrate the global market is measured by their success rate. Because they have a considerable potential to influence the treatment of psoriasis, the emergence of innovative drug delivery systems based on contemporary topical products in the market is eagerly anticipated. These are the main factors that are expected to propel the market for psoriasis treatments upward in the next years.
The Psoriasis Treatment Market News
- In March 2023, in India, Eli Lilly launched a new drug, Copellor, for the treatment of moderate-to-severe plaque psoriasis. This drug was specially designed to target the protein that plays an important role in maintaining and triggering inflammation in psoriasis. This drug was prescription medicine and was only used by dermatologists and under medical supervision, such as for psoriasis.
- In August 2023, for the treatment of 40 million Americans who suffered from psoriasis, Arctiva Wellness, the skincare company, launched an eczema product line and breakthrough psoriasis. This newly launched product line involves two steroid-free and clean skin care products that are the first to merge clinically approved active elements with exclusive HYDROSURF natural and glycolipid technology.
- In September 2023, for the treatment of eczema-prone skin, rosacea and psoriasis disease, Derma Solutions launched new skin product No 7. This product was ideal for all skin problems.
Market Segmentation
By Drug Class
- Interleukins
- TNF Inhibitors
- Others
By Treatment Type
- Biologic Drugs
- Small Molecule Systemic Drugs
- Tropical Therapies
By Type
- Psoriatic Arthritis
- Plaque Psoriasis
- Others
By Route of Administration
- Oral
- Parenteral
- Topical
By Distribution Channel
- Hospital Pharmacies
- Online pharmacies
- Retail Pharmacies
Buy this Research Report@ https://www.precedenceresearch.com/checkout/2034
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308