Single-Dose Gene Therapy Market Size and Forecast 2025 to 2034
Single-dose gene therapy market sees rapid growth driven by advancements in genetic engineering and increasing demand for lasting cures. The single-dose gene therapy market is growing as biopharmaceutical companies are focusing on developing new gene therapies that last longer. The rising approval for AAV-based therapies and the increasing prevalence of rare diseases further support market growth.
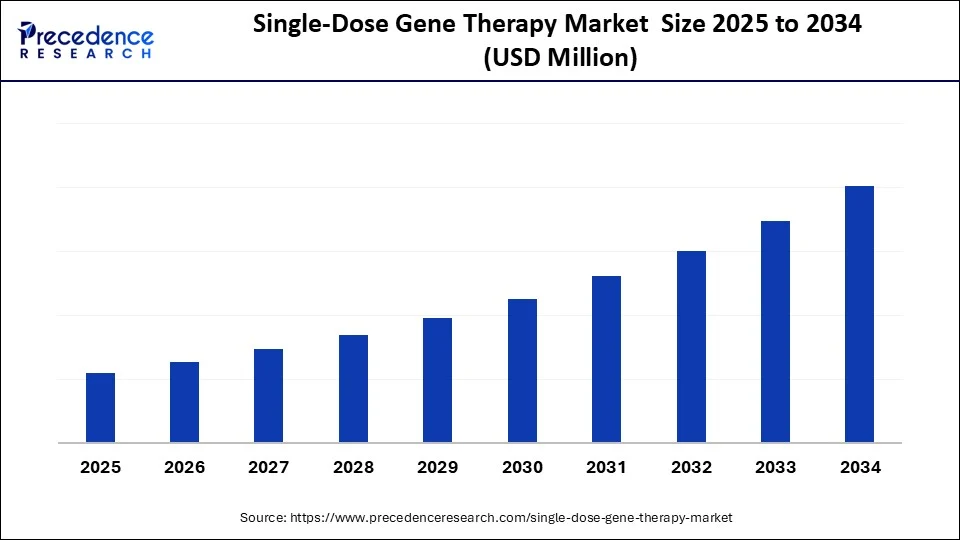
Single-Dose Gene Therapy MarketKey Takeaways
- North America led the single-dose gene therapy market in 2024.
- Asia Pacific is expected to expand at the fastest CAGR in the market between 2025 and 2034.
- By therapy type, the in vivo gene therapy segment held the largest market share in 2024.
- By therapy type, the ex vivo gene therapy segment is anticipated to grow at a remarkable CAGR between 2025 and 2034.
- By vector type, the AAV-based vectors segment captured the biggest market share in 2024.
- By vector type, the non-viral vectors (plasmid, nanoparticle) segment is expected to expand at a notable CAGR over the projected period.
- By disease area, the neuromuscular disorders (e.g., SMA) segment contributed the highest market share in 2024.
- By disease area, the hematologic disorders (e.g., Hemophilia A/B) segment is expected to expand at a notable CAGR over the projected period.
- By patient type, the pediatric segment accounted for the highest market share in 2024.
- By patient type, the adults segment is expected to expand at a notable CAGR over the projected period.
- By route of administration, the intravenous (IV) segment held the major market share in 2024.
- By route of administration, the intramuscular / localized delivery segment is expected to expand at a notable CAGR over the projected period.
- By end user, the hospitals and specialty clinics segment contributed the largest market share in 2024.
- By end user, the contract development and manufacturing organizations (CDMOs) segment is expected to expand at the fastest CAGR over the projected period.
Impact of AI on the Single-Dose Gene Therapy Market
Artificial Intelligence significantly transforms the single-dose gene therapy market. AI is becoming particularly critical in the engineering of more effective, safer viral vectors used to deliver therapeutic genes. AI algorithms analyze vast datasets of genomics and proteomics to identify potential gene therapy targets, ultimately shortening development time by nearly 40%. AI also helps researchers with patient response prediction, optimizing clinical trials, and patient selection. Furthermore, AI-enabled platforms such as NVIDIA's BioNeMo, which were originally focused on simulating protein folding and gene interactions, are being used in preclinical validation for gene therapies. These platforms allow biotechnology companies to move from the traditional method of developing gene therapies based on trial and error.
Market Overview
The single-dose gene therapy market refers to the segment of gene therapy in which a single administration of a viral or non-viral vector delivers a therapeutic gene to correct or compensate for a defective or missing gene. These therapies aim to provide long-term or permanent clinical benefits with one dose, primarily using adeno-associated virus (AAV), lentivirus, or emerging delivery platforms. Applications span rare genetic disorders, neuromuscular diseases, hematology, ophthalmology, and metabolic conditions. An increased need for curative therapies, specifically for rare diseases, is driving market growth. Continued advancements in vector designs, support from regulatory bodies, and rising clinical trials are boosting the growth of the market.
Single-Dose Gene Therapy Market Growth Factors
- Increasing Incidence of Rare Genetic Disorders: The growing prevalence of rare and inherited diseases leads to more single-dose gene therapies, which offer durable relief to patients with fewer treatment encumbrances for the patient/caregiver.
- Advances in Vector Technology: Advancements in AAV and lent viral vector engineering increase safety, accuracy, and gene expression duration, making therapies safer, more durable, and applicable for more diseases.
- Supportive Regulatory Frameworks: Innovative acceleration pathways and orphan drug designations from regulatory agencies (e.g., FDA, EMA) increase rapid development and commercialization of innovative gene therapy.
- Increased Investment and Partnership: Massive investment in biotech, strategic partnerships, and acquisitions enable R&D programs to advance more quickly from clinical trials to commercialization
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Therapy Type, Vector Type, Disease Area, Patient Type, Patient Type, Route of Administration, End-User and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Strong Regulatory Support and Clinical Trial Results
One of the major factors driving the growth of the single-dose gene therapy market is the increased regulatory support from global authorities and the proven effectiveness of gene therapies in treating a range of conditions. Single-dose gene therapies provide physicians and specialists with the potential to "cure" genetic disorders with a single infusion of treatment, thereby potentially reducing the burden of chronic treatment. The FDA recognized the possible cure potential of single-dose therapies. Thus, it is fast-tracking the development and approval of these therapies.
- In September 2024, Vironexis Biotherapeutics received FDA clearance for its Investigational New Drug (IND) application from the U.S. FDA for VNX-101, its first gene therapy product candidate, for the treatment of CD19+ acute lymphoblastic leukemia. This marks the beginning of single-dose gene therapy in oncology.
(Source: https://vironexis.com)
In India, the Indian Council of Medical Research (ICMR), the Department of Biotechnology (DBT), and CDSCO developed and issued guidance for national guidelines to consider ethical and scientific development together and show strong institutional support that facilitates research, as well as streamline clinical research approvals, which supports the market growth. Additionally, the increasing government funding for expanding the pipeline of gene therapies drives the market's growth.
Restraint
Durability Concerns and High Cost
While single-dose gene therapies have the potential for long-term or even curative effects, the durability of these effects remains a key question over the long term. Many of these therapies have only a few years of clinical data, limiting the understanding of their longevity. The Institute for Clinical and Economic Review (ICER), in its recent white paper (April 2024), noted uncertainty around the long-term benefit of these therapies and posed the question of whether one-time treatment could provide multi-year protection or consistent symptom resolution.
In some cases, the therapeutic effect of single-dose gene therapy may diminish over time, requiring additional intervention. This means that uncertainty around long-term efficacy continues to serve as a limiting factor in the overall implementation of these therapies, particularly in systems focused on value-based care. Moreover, the high costs associated with these therapies limit patient access and create challenges for small healthcare organizations.
Opportunity
High Unmet Medical Needs and Technological Innovations
One of the major opportunities in the single-dose gene therapy market lies in the considerable unmet medical needs for rare diseases. Single-dose gene therapies address these unmet medical needs. The opportunities are vast as the global landscape for clinical trials expands. The FDA approved several gene therapy INDs (investigational new drugs) in 2024, with expanding trials in ophthalmic and neuromuscular disorders, among others. Technological advances open up new growth avenues for the market. Advancements in gene editing and drug delivery systems are improving the safety and efficacy of these therapies while enhancing patient outcomes.
Therapy Type Insights
Why Did the In Vivo Gene Therapy Segment Dominate the Market in 2024?
The in vivo gene therapy segment dominated the single-dose gene therapy market with the largest share in 2024. This is mainly due to the increased adoption of this therapy because it offers the greatest convenience. In vivo gene therapy enables the direct delivery of therapeutic genes into a patient's body. It also minimizes issues associated with complicated manipulation of cells, proving beneficial for rare genetic diseases such as spinal muscular atrophy (SMA).
The ex vivo gene therapy is considered to be the fastest-growing segment. The growth of the segment is attributed to the rising prevalence of hematological and immune-related conditions. Ex vivo gene therapy involves changing a person's cells outside the body before returning them to the patient; thus, it provides a stronger level of patient safety and control. Recent advancements in gene editing, including CRISPR-Cas9, are enhancing the controls and precision of therapy, creating opportunities for personalized medicine to treat more complex diseases.
Vector Type Insights
Which Vector Type Segment Dominate the Market in 2024?
The AAV-based vectors segment dominated the single-dose gene therapy market in 2024. Non-pathogenicity, long-term gene expression, and the ability to selectively target tissues make AAVs a preferred delivery system in gene therapies. AAVs have been instrumental in successful outcomes for the treatment of SMA and inherited retinal diseases, so AAVs remain at the forefront of gene therapy delivery.
The non-viral vector segment (e.g., plasmids and non-viral nanoparticle vectors) is expected to grow at the fastest rate in the upcoming period, owing to its lesser immunogenicity, ease of scalability in manufacturing, and lighter regulatory burden, and provide a safer alternative vector for gene delivery compared to viral vectors. Non-viral vectors are gaining traction for their wide application in cancer and metabolic diseases. Advancements in nanoparticle engineering and electroporation techniques are generating more clinical interest and investment.
Disease Area Insights
What Made Neuromuscular Disorders the Dominant Segment in the Single-Dose Gene Therapy Market in 2024?
Neuromuscular disorders, particularly SMA, dominated the market, accounting for the largest revenue share in 2024. This is mainly due to the increased prevalence of neurological disorders and high unmet medical needs. Zolgensma, the only available single-dose AAV gene therapy with proven clinical benefit, has established a precedent in the pediatric population with SMA. These product areas are expected to continue thriving due to the large unmet medical need and the life-changing outcomes achievable through early gene intervention.
The hematologic disorders, including Hemophilia A and B, segment is expected to grow at the fastest rate during the forecast period. This is mainly due to the increasing prevalence of bleeding disorders, blood cancers, and bone marrow disorders. There is an increase in the number of late-stage trials, some of which have recently been approved, while others demonstrate the possibility of single-dose approved therapies that can sustain factor expression with fewer bleeding episodes. As soon as gene therapies demonstrate more durable alternatives to invasive and chronic treatments, this area is set to expand rapidly.
Patient Type Insights
Why Did the Pediatric Segment Dominate the Market in 2024?
The pediatric segment dominated the single-dose gene therapy market with the largest share in 2024. This is due to the increased prevalence of genetic disorders like SMA among pediatrics, where early intervention is critical and can substantially change the progression of the disease. The FDA and EMA have been providing funding for pediatric orphan drugs, simplifying the paths to drug approval and promoting the development of therapies. The life-changing therapeutic impact on infants and children has further enhanced investors' and developers' clinical and commercial focus on this patient population.
The adult segment is expected to grow at a significant rate as gene therapies targeting adult-onset conditions, such as hemophilia, certain cancers, and inherited retinal disorders, progress more quickly through clinical trials. Technology has enhanced the ability of vectors to target specific spots in the genome with enough durability for adult-onset disease. Regulatory agencies are approving adult indications with increased frequency and are encouraging the development of potential adult indications through targeted gene therapy.
Route of Administration Insights
How Does the Intravenous (IV) Segment Dominate the Market in 2024?
The intravenous (IV) segment dominated the market with a major revenue share in 2024. The segment's dominance is mainly attributed to the increased demand for targeted therapies. IV administration can be performed according to AAV vector specifications and has become a standard practice in clinical settings for therapies targeting SMA, hemophilia, and certain metabolic disorders. There are several advantages to IV delivery that support its dominance in delivery routes for AAV and lentivirus vector therapies, including the rapid onset of action and high efficacy in delivering therapeutic genes.
The intramuscular/localized delivery segment is growing at the fastest CAGR in the coming years, as it is gaining popularity as a "safer" and more directed option. Localized delivery reduces potential systemic exposure and immune response and consequently may improve safety. As the local site of delivery vector and expression technology advances, the adoption will progress more rapidly in precision therapy contexts.
End User Insights
Why Did the Hospitals & Specialty Clinics Segment Dominate the Single-Dose Gene Therapy Market in 2024?
The hospitals & specialty clinics segment dominated the market with the biggest share in 2024. This is mainly due to the increased patience in these settings, as they are considered the primary point of contact. Patient delivery for single-dose gene therapies is primarily based in hospitals and specialty clinics, accounting for the majority of patient treatments for pediatric and rare genetic disorders. These facilities have the infrastructure to support gene delivery, patient monitoring and emergent situations. The therapies are primarily provided and delivered within highly supervised clinical settings, which bolsters segmental growth.
The contract development & manufacturing organizations (CDMOs) segment is expected to grow at the fastest CAGR during the studied period due to the rising outsourcing trend. Because of the complexity of the manufacturing process and scaling up issues, biopharmaceutical companies are outsourcing gene therapy manufacturing to CDMOs. CDMOs have experience with a variety of critical issues, including vector development, regulatory approval and compliance, and GMP standards. With biotech companies increasingly outsourcing the development of their therapeutics, the niche space that CDMOs occupy continues to show strong growth.
Regional Insights
What Made North America the Leader in the Single-Dose Gene Therapy Market?
North America dominated the market by capturing the largest share in 2024. This is mainly due to its advanced biotechnology infrastructure, rapid rate of FDA approvals, and increased partnerships between biopharmaceutical and healthcare organizations aimed at boosting the production of gene therapies. The region boasts high levels of public awareness and favorable reimbursement policies, which support the market's growth. Increased partnerships between biotechnology companies and academic institutions, as well as the growing demand for targeted therapies for rare and genetic diseases, contribute to regional market growth.
The U.S. stands out in the North American market due to the increased number of new clinical trials and FDA approvals for single-dose gene therapies for hemophilia and spinal muscular atrophy. Increasing investment in biotech research initiatives also contributes to market growth. The National Institute of Health's Accelerating Medicines Partnership (AMP) and Biomedical Advanced Research and Development Authority's initiatives are fostering an encouraging environment for biotechnology research. In 2024, the FDA successfully reviewed and approved several Investigational New Drug (IND) applications, driving the growth of the market.
European Single-Dose Gene Therapy Market Trends
Europe is the second-largest market. The reasons behind Europe's position as the second-largest region are its strong healthcare infrastructure, a strong regulatory environment, and substantial funding to develop treatments for rare diseases. The rising support from the European Medicines Agency (EMA) and the increase in the number of gene therapy clinical trials in European countries, such as Germany, France, and the UK, continue to foster market expansion. Germany is a major player in the market. The country has a strong biotechnology ecosystem. The rising spending on R&D and partnerships between academic institutions and pharmaceutical companies support market growth.
Asia Pacific Single-Dose Gene Therapy Market Trends
Asia Pacific is expected to grow at the fastest rate during the projection period. This is mainly due to improved access to advanced healthcare, investment in biotech innovation, and a rise in the prevalence of genetic disorders. Governments across the region are investing in programs devoted to rare diseases and reforming regulations for clinical studies. Countries such as China, India, Japan, and South Korea have established extensive centers that foster gene therapy studies. Additionally, the region has become a hub for clinical studies, thereby supporting regional market growth.
China is leading the way with investments in gene therapy research and development as well as precision medicine. The National Medical Products Administration (NMPA) has made efforts to expedite IND approvals for regulators. The presence of a well-established biopharmaceutical sector and the growing pipeline of gene therapies further boost the market's growth.
Single-Dose Gene Therapy Market Companies

- Option Care Health
- InfuCare Rx
- Coram CVS (now part of Option Care)
- KabaFusion
- BioScrip (now part of Option Care)
- Amerita (a PharMerica company)
- United Infusion
- Soleo Health
- Paragon Healthcare
- PromptCare
- ContinuumRx
- CareCentrix
- Chartwell Pennsylvania
- Intramed Plus
- AlayaCare
- ClearSky Health
- Healix Infusion Therapy
- Nufactor (a FFF Enterprises company)
- PharMerica Infusion Services
- Infusion Associates
Recent Developments
- In May 2025, Rocket Pharmaceuticals, Inc. announced an update related to RP-A501, its investigational gene therapy for Danon disease.
(Source:https://ir.rocketpharma.com)
- In April 2024, Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) has approved BEQVEZ (fidanacogene elaparvovec-dzkt) for the treatment of adults with moderate to severe hemophilia B. BEQVEZ is an adeno-associated virus (AAV)-based gene therapy intended to introduce in the transduced cells a functional copy of the FIX gene encoding a high-activity FIX variant.
(Source:https://www.pfizer.com)
Segments Covered in the Report
By Therapy Type
- In Vivo Gene Therapy
- Gene delivered directly into the patient's body.
- Ex Vivo Gene Therapy
- Cells modified outside the body and reintroduced (e.g., CAR-T, hematopoietic cells).
By Vector Type
- AAV-based Vectors
- Lentiviral Vectors
- Retroviral Vectors
- Non-Viral Vectors (plasmid, nanoparticle)
By Disease Area
- Neuromuscular Disorders (e.g., SMA)
- Ophthalmology (e.g., inherited retinal diseases)
- Hematologic Disorders (e.g., Hemophilia A/B)
- Metabolic Disorders (e.g., OTC deficiency, Fabry)
- Cardiovascular Disorders
- CNS Disorders (e.g., MLD, Batten disease)
- Others (e.g., rare pediatric diseases)
By Patient Type
- Pediatric (Largest)
- Adult
By Route of Administration
- Intravenous (IV)
- Intrathecal (IT)
- Subretinal (for ocular therapies)
- Intramuscular / Localized Delivery
By End-User
- Hospitals & Specialty Clinics
- Academic & Research Institutions
- Contract Development & Manufacturing Organizations (CDMOs)
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting