Anti-Thyroid Peroxidase Antibody Test Kit Market Size and Forecast 2025 to 2034
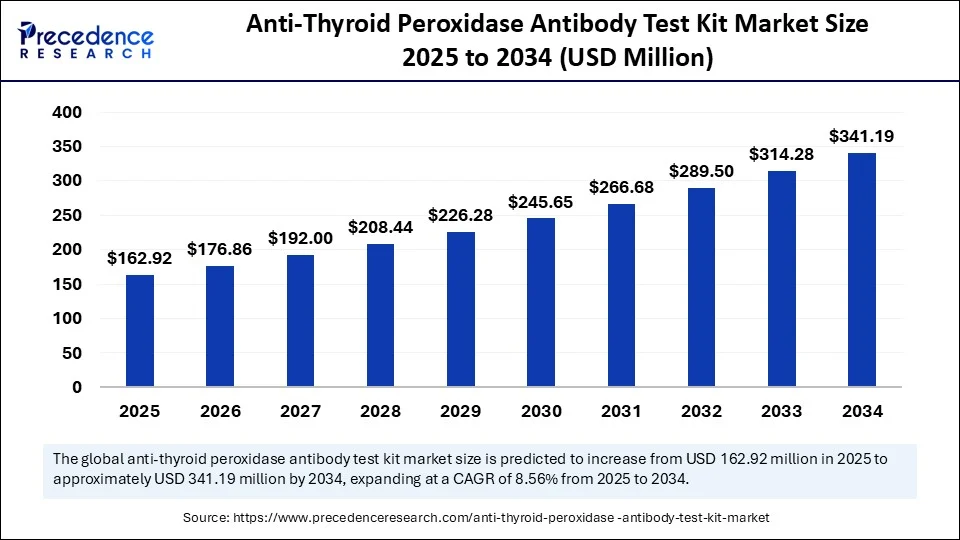
The global anti-thyroid peroxidase antibody test kit market size accounted for USD 150.07 million in 2024 and is predicted to increase from USD 162.92 million in 2025 to approximately USD 341.19 million by 2034, expanding at a CAGR of 8.56% from 2025 to 2034. The market for anti-thyroid peroxidase antibody test kits continues to grow due to an increasing prevalence of autoimmune thyroid disease, advances in diagnostic technology, and increased health care spending and awareness, leading to a strong upward trajectory for the market.
Anti-Thyroid Peroxidase Antibody Test Kit Market Key Takeaways
- In terms of revenue, the global anti-thyroid peroxidase antibody test kit market was valued at USD 150.07 million in 2024.
- It is projected to reach USD 341.19 million by 2034.
- The market is expected to grow at a CAGR of 8.56% from 2025 to 2034.
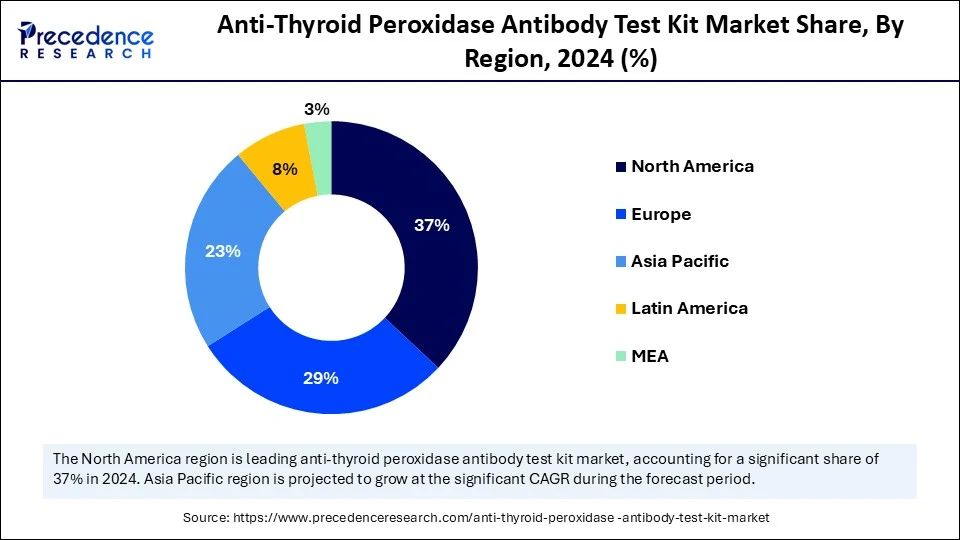
- North America led the anti-thyroid peroxidase antibody test kit market with the highest revenue share of 37% in 2024.
- Asia Pacific is estimated to expand at the fastest CAGR in the market between 2025 and 2034.
- By technology, the enzyme-linked immunosorbent assay (ELISA) segment held 40% of the market share in 2024.
- By technology, the chemiluminescence immunoassay (CLIA) segment is anticipated to grow at a remarkable CAGR between 2025 and 2034.
- By test type, the quantitative test kits segment captured the biggest market share of 58% in 2024.
- By test type, the semi-quantitative test kits segment is expected to expand at a notable CAGR over the projected period.
- By sample type, the serum segment captured the highest market share of 65% in 2024.
- By sample type, the dried blood spots (DBS) segment is expected to expand at a notable CAGR over the projected period.
- By end user, the diagnostic laboratories segment held the major market share of 43% in 2024.
- By end user, the point-of-care settings segment is expected to expand at a notable CAGR over the projected period in the anti-thyroid peroxidase antibody test kit market.
- By distribution channel, the distributors/wholesalers segment held a largest market share of 47% in 2024.
- By distribution channel, the online platforms segment is expected to expand at a notable CAGR over the projected period.
- By application, the autoimmune thyroid disease diagnosis segment generated the major market share of 52% in 2024.
- By application, the pregnancy-related thyroid monitoring segment is expected to expand at a notable CAGR over the projected period.
How Does AI Improve Anti-Thyroid Peroxidase Antibody Test Reliability and Diagnostic Accuracy?
Artificial intelligence is making significant strides in improving the accuracy of anti-thyroid peroxidase antibody test kits with recent innovations in evaluating thyroid disease. A recent literature review highlights how AI is being used to enhance thyroid diagnostics, from discerning malignant from benign nodules and for monitoring patient prognostics to the development of diagnostic techniques for finding thyroid disease by evaluating serology, imaging, and physician office notes together as one data set.
In addition to this, AI is being used as an innovative model of classifying not only the stage but also the risk of thyroid cancer with accuracy exceeding 90% while also reducing the time for clinician preparation by half.These innovations are a harbinger of how AI will play an increasingly significant role in the interpretation of anti-TPO test results in a new and smarter diagnostic environment and greatly propel the anti-thyroid peroxidase antibody test kit market. (Source: https://ecancer.org)
U.S. Anti-Thyroid Peroxidase Antibody Test Kit Market Size and Growth 2025 to 2034
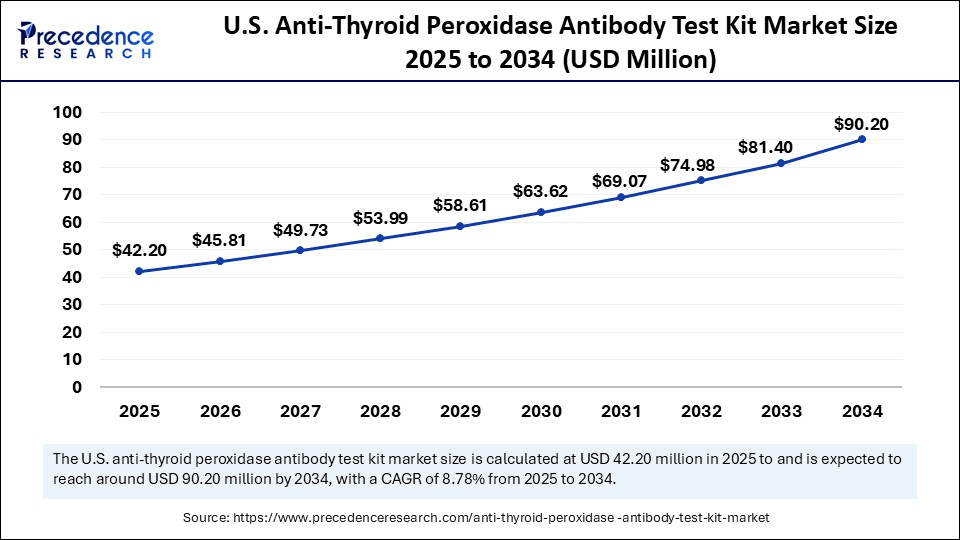
The U.S. anti-thyroid peroxidase antibody test kit market size was exhibited at USD 38.87 million in 2024 and is projected to be worth around USD 90.20 million by 2034, growing at a CAGR of 8.78% from 2025 to 2034.
Why Is North America the Most Important Region in the Anti-Thyroid Peroxidase Antibody Test Kit Market for 2024?
The North America region will continue to dominate, as it has the most advanced health care systems, diagnostic infrastructures widely available, uptake of the immunoassay-based technologies such as ELISA and chemiluminescence, it has the most robust research and development activity, and regulatory environments that foster problem-solving and innovative diagnostics. Also, patients now aware of autoimmune thyroid disease are increasingly requesting testing solutions to obtain accurate results. Overall, North America will remain the most developed and lucrative marketplace for anti-TPO test kits globally.
The U.S.is the most developed and largest area due to the number of endocrinology specialists, point-of-care adoption, plentiful levels of public funding and private fund investments, and large public and private health care policies. The key testing methods as immunoassays using chemiluminescence, are used in both hospital and lab settings, driven by a demand for precision testing and diagnostics. In addition, testing automation will continue to improve along with changes to rapid tests and point-of-care potential, such that the United States is the central location of the infrastructure and uptake of anti-TPO test kits in North America.
Why Is Asia Pacific Projected To Be the Fastest-Growing Region During the Forecast Period?
Asia Pacific is projected to be the fastest-growing region, mainly owing to the increasing prevalence of thyroid disorders, expanding healthcare coverage, and rising expenditure on diagnostics. Many countries in the region are upgrading laboratory infrastructure and are increasingly using immunoassay-based testing techniques. Much of the growth is taking place in urban or semi-urban areas due to increased access to healthcare and the growing awareness of autoimmune diseases, leading to increased demand for reliable anti-TPO kits.
China is driving much of the growth in Asia Pacific, as it addresses the gap in a structured manner as a result of the massive healthcare drive in the region that includes public health funding initiatives, a rapid rise in diagnostic lab services, and a focus on screening thyroid diseases. The uptake of immunoassay techniques, such as ELISA and chemiluminescence, is contributing to the early detection of the problems associated with thyroid screening on a scale now not previously possible. The Chinese government is continuing efforts to fund these awareness and screening programs, as well as increasing its production capacity, making it the most important player in the rapidly advancing anti-TPO test kit market in the region.
Market Overview
The anti-thyroid peroxidase antibody test kit market refers to the global industry involved in the manufacturing, distribution, and use of diagnostic kits designed to detect anti-thyroid peroxidase antibodies in human serum or plasma. These antibodies are often elevated in autoimmune thyroid disorders, including Hashimoto's thyroiditis and Graves' disease, and serve as a key diagnostic biomarker. The market encompasses various testing technologies (e.g., ELISA, CLIA), test types (quantitative and qualitative), settings (clinical laboratories, hospitals, research labs), and user segments (healthcare providers, research institutions). These kits are increasingly being integrated into routine thyroid function panels in both developed and emerging healthcare ecosystems.
Anti-Thyroid Peroxidase Antibody Test Kit Market Growth Factors
- Increasing Incidences of Autoimmune Thyroid Disorders: Emerging rates of autoimmune thyroid conditions, such as Hashimoto's thyroiditis and Graves' disease, due to altered iodine intake, low nutrient intake for selenium and vitamin D, are increasing the need for early and accurate diagnosis. This is helping to drive the growth of TPO antibody test kit utilization.
- Increased Awareness Around Thyroid Health: Public health campaigns, along with general increases in patient awareness of the symptoms of thyroid-related issues, are leading to routine screening requests for potential disorders. These developments indicate a more favorable market for diagnostic kits.
- Developments in Diagnostic Technologies: New developments in the areas of assay sensitivity, automation, and laboratory infrastructure mean that TPO antibody detection can occur with greater accuracy and efficiency, stimulating clinical use of TPO antibody diagnostic kits.
- Increasing Elderly Population: There has been a significant increase in the older population, who are more susceptible to autoimmune and thyroid conditions, increasing the demand for thyroid-related diagnostic testing.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 341.19 Million |
| Market Size in 2025 | USD 162.92 Million |
| Market Size in 2024 | USD 150.07 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 8.56% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Technology, Test Type, Sample Type, End User, Distribution Channel, Application, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Why Is The Increasing Frequency of Autoimmune Thyroid Disorders Providing Demand for Test Kits?
One of the most notable factors responsible for the increased demand for anti-thyroid peroxidase antibody test kits market is the increasing incidence of autoimmune thyroid diseases, namely, Hashimoto's thyroiditis and Graves' disease. Autoimmune thyroid diseases are some of the most common autoimmune disorders worldwide and are specifically associated with high levels of anti-TPO antibodies. As per the American Thyroid Association, there are approximately 20 million Americans living with some form of thyroid disease, with almost 60% undiagnosed. (https://www.thyroid.org/media-main/press-room/) Increasing exposure to pollutants such as fungicides and industrial chemicals such as polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), and bisphenol A (BPA) is linked to higher thyroid autoantibody levels and related inflammation.
The high prevalence presents ongoing demand for early and accurate diagnosis, encouraging hospitals and labs to add anti-TPO testing to their routine thyroid panels. Moreover, awareness is growing that testing and screening in primary care is leading to an increase in reliable test kit demand, motivating manufacturers to increase production capacity and enhance test sensitivities for differentiated, competitive testing.
Restraint
How Is Insufficient Diagnostic Capacity in Developing and Under-Resourced Regions Impacting Market Growth?
The significant restraint within the anti-thyroid peroxidase antibody test kit market is the underdeveloped diagnostic capacity of many developing and under-resourced regions. Many regions, including, but not limited to, some parts of Asia, Africa, and Latin America, have less-than-sufficient laboratory facilities, little automation, and a lack of personnel specializing in thyroid care among healthcare professions.
The Undiagnosed Diseases Network International reported that 350 million people live with an undiagnosed disease worldwide, or with poorly supported access to at least essential diagnostic services.This gap limits the timely diagnosis of autoimmune thyroid disease and precludes the use of specific tests, such as testing for anti-TPO antibodies. The aforementioned conditions pose a challenge for manufacturers and suppliers entering a high unmet need but under-skilled target market, limiting the feasibility of scaling production, education, and support to ensure that patients facing a health issue receive sustainable and consistent diagnostic service. (Source:https://www.udninternational.org)
Opportunity
Why Is Expanded Prenatal Thyroid Screening Providing New Opportunities for Growth?
As thyroid screening is steadily being prioritized during pregnancy, this represents a noteworthy opportunity for providers of anti-thyroid peroxidase (anti-TPO) antibody test kits. Elevated anti-TPO antibodies are a marker of thyroid autoimmunity and represent a significant risk factor for miscarriage, preterm birth, and developmental complications in infants. Understanding this, many governments and organizations have started to include thyroid screening during pregnancy as part of routine care.
For instance, India's National Health Mission has established a program that supports thyroid testing during pregnancy under the RMNCH+A initiative to reduce maternal and neonatal morbidity and mortality rates. Additionally, recent clinical guidelines have called for targeted thyroid screening during early pregnancy in countries like the U.S. and the U.K. The growing prevalence of screening protocols means demand for reliable anti-TPO test kits is increasing and has led to several diagnostic companies developing pregnancy-specific testing panels and introducing their products into the larger maternal healthcare market.
Technology Insights
Why Enzyme-Linked Immunosorbent Assay (ELISA) Is the Dominant Technology Segment?
The ELISA segment was by far the most dominant technology in the anti-thyroid peroxidase antibody test kit market in 202 due to its unique advantages such as high sensitivity, specificity, and cost-effectiveness. Further, ELISA has become ubiquitous in both research and clinical laboratories, thus very commonly employed to detect anti-TPO antibodies. The ELISA's capacity to process large batches of samples with reproducible performance continues to give it gnarly momentum within the diagnostics market. Added to this, ELISA kits can be used on automated systems, and therefore can be incorporated in a routine manner within centralized diagnostic laboratory environments.
The Chemiluminescence Immunoassay (CLIA) segment is expected to continue growing at the fastest rate over the forecast period. CLIA assays have higher sensitivity and faster turnaround times than conventional methods, which has established its uptake in more advanced healthcare environments. CLIA has the potential to be applied on very high-throughput platforms while delivering accurate quantification, furthering interest amongst those users requiring fast diagnoses. The need for automated and efficient tools in diagnostics has led to growing demands for CLIA assays to be used in the future years.
Test Type Insights
Which Test Type Dominates the Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024?
The quantitative test kits segment was the leading segment with respect to sales in 2024, due to their accuracy when quantifying exact levels of antibody levels. Quantitative kits are typically used to track disease progression and response to treatment, especially in autoimmune thyroid conditions like Hashimoto's thyroiditis, and are acceptable within clinical settings due to their ability to deliver numerical values. Because they yield specific values, endocrinologists can rely on these values to accurately assess small changes to thyroid antibodies.
The semi-quantitative test kits segment will grow at the fasted rate during the forecast period, as these kits are both less expensive and easier to use, and more likely to be used in resource-limited or initial screening settings. The opportunity to test for autoimmune thyroid disorders more quickly at low-cost settings, and expanded awareness of autoimmune thyroid disorders, will increase in demand for these kits.
Sample Type Insights
Why Does the Serum Segment Dominate Sample Types in the Anti-Thyroid Peroxidase Antibody Test Kit Market?
The serum segment accounted for the largest share of the anti-thyroid peroxidase antibody test kit market in 2024, owing to its high reliability and established use for clinical diagnosis. Serum tests are widely recognized for their accuracy in finding anti-thyroid peroxidase antibodies, so they are favored methods of diagnostic laboratories and hospitals. The standardization of serum collection and testing contributes to its continued market presence in thyroid antibody diagnostics.
The dried blood spots (DBS) segment is forecasted to grow at the fastest rate in the forecast period. DBS offers convenience in terms of sample collection, storage, and transport, especially in remote places or resource-limited settings. Its minimally invasive nature and increasing use in home-based and decentralized testing environments also play a role in its growing popularity in the thyroid diagnosis market.
End-User Insights
Which End-User Segment Dominates the Anti-Thyroid Peroxidase Antibody Test Kit Market in 2024?
The diagnostic laboratories segment captured the largest share of the market in 2024. Through technology and trained personnel, diagnostic laboratories offer advanced testing and are the ideal setting for conducting anti-thyroid peroxidase antibody tests. High volume processing for reliable and accurate test results is the most significant contributing factor to maintaining their dominance in the market.
The point-of-care settings segment is expected to grow at the fastest rate during the forecast period. The growing demand for rapid testing, especially in primary care clinics and in remote areas, is driving up acceptance rates. The simplicity that offers quick results and little infrastructure is creating more opportunities for the test kits to be used outside the pace of ta traditional laboratory setting.
Distribution Channel Insights
Why Distributors/Wholesalers Are the Most Dominant in the Anti-Thyroid Peroxidase Antibody Test Kit Market?
The distributors/wholesalers segment led the market in 2024. These intermediaries enable continuous supply and access to test kits to ensure hospitals, laboratories, and clinics maintain appropriate medical inventories. The market position of these intermediaries remains strong because they have wide distribution channels in multiple locations and strong partnerships to ensure their products are available.
The online platforms segment is expected to develop the fastest over the forecast period. Their development is driven by increased reliance on the digital purchasing process and delivery methods, and the convenience of browsing updated test kits to purchase, especially for smaller health care providers and end users in remote geographical areas.
Application Insights
Why Does the Autoimmune Thyroid Disease Diagnosis Segment Dominate the Market in 2024?
The autoimmune thyroid disease diagnosis segment was the foremost application in 2024. The frequent diagnosis of autoimmune thyroid diseases, including Hashimoto's thyroiditis and Graves' disease, makes autoimmune tests well-liked. The tests aid healthcare partners in the early detection of TPO antibodies, which are key markers in the diagnosis of autoimmune thyroid diseases.
The pregnancy thyroid monitoring enzyme immunoassay kit segment is forecasted to have the largest growth in the anti-thyroid peroxidase antibody test kit market during the forecast period. The increasing awareness of the importance of thyroid dysfunction in maternal and fetal health drives physicians to recommend screening for maternal thyroid dysfunction. Early detection with screening and monitoring tests can lead to early intervention benefits that prevent or ameliorate complications during pregnancy and result in better prenatal health outcomes.
Anti-Thyroid Peroxidase Antibody Test Kit Market Companies

- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Siemens Healthineers
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd.
- Danaher Corporation (Beckman Coulter)
- DiaSorin S.p.A
- Ortho Clinical Diagnostics
- Merck KGaA
- EUROIMMUN AG (a PerkinElmer company)
- Tosoh Corporation
- Biorbyt Ltd
- Abcam plc
- Inova Diagnostics, Inc.
- Seramun Diagnostica GmbH
- Trinity Biotech
- MyBioSource, Inc.
- Creative Diagnostics
- IBL-America
- Biocheck, Inc.
Segments Covered in the Report
By Technology
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Direct ELISA
- Indirect ELISA
- Chemiluminescence Immunoassay (CLIA)
- Radioimmunoassay (RIA)
- Immunofluorescence Assay (IFA)
- Others
- Turbidimetric Immunoassay
- Lateral Flow Assay
By Test Type
- Qualitative Test Kits
- Quantitative Test Kits
- Semi-Quantitative Test Kits
- Others
By Sample Type
- Serum
- Plasma
- Whole Blood
- Others
- Dried Blood Spots (DBS)
By End User
- Hospitals
- Diagnostic Laboratories
- Independent Labs
- Chain Labs
- Research & Academic Institutes
- Point-of-Care Settings
- Others
- Ambulatory Surgical Centers
- Physician Clinics
By Distribution Channel
- Direct Sales
- Distributors/Wholesalers
- Online Platforms
- Others
By Application
- Autoimmune Thyroid Disease Diagnosis
- Hashimoto's Thyroiditis
- Graves' Disease
- Thyroid Dysfunction Screening
- Pregnancy-Related Thyroid Monitoring
- Research Applications
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting