Dental Bone Grafts Substitutes Market Size and Forecast 2025 to 2034
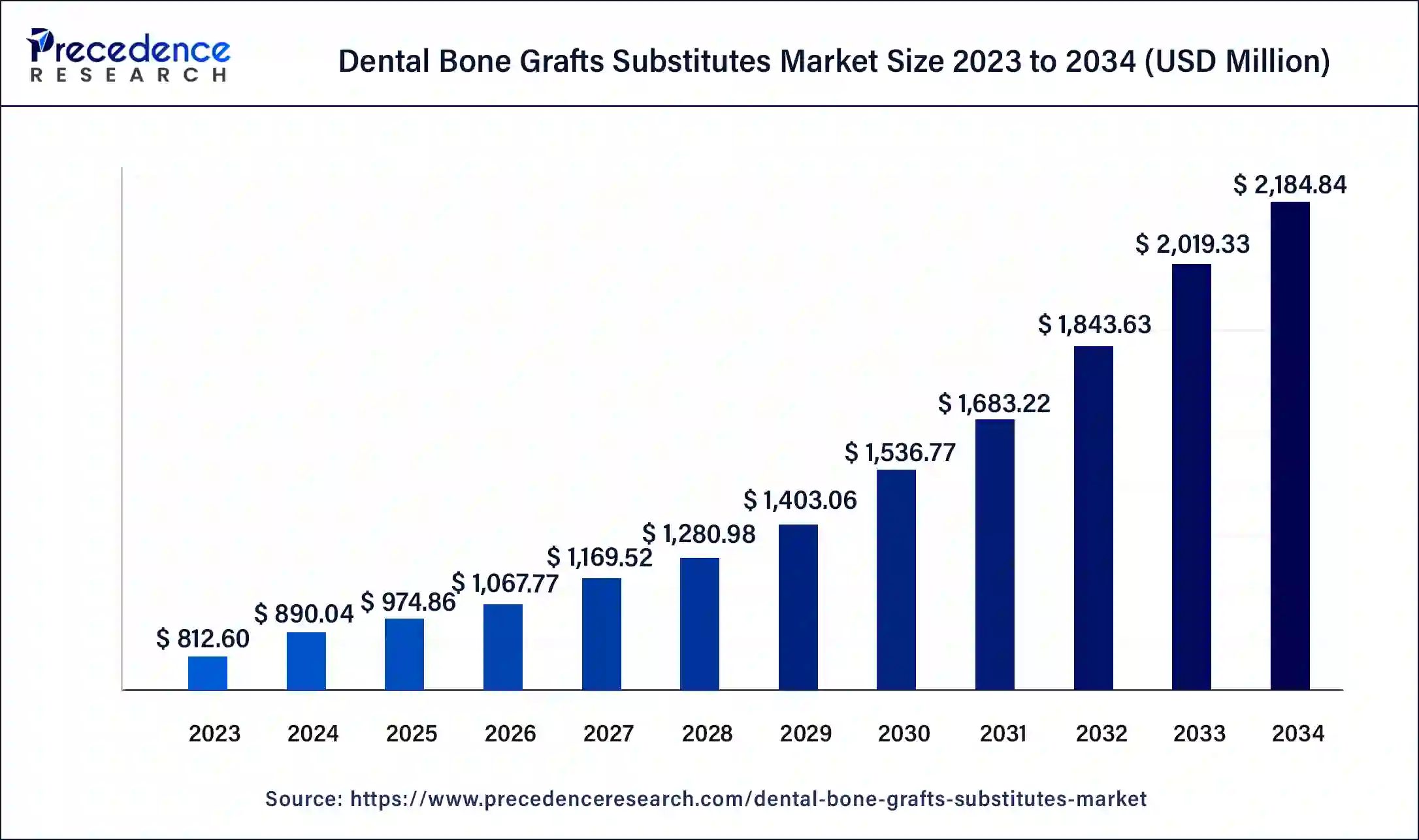
The global dental bone grafts substitutes market size accounted at USD 890.04 million in 2024 and is predicted to reach around USD 2,184.84 million by 2034, growing a notable CAGR of 9.40% from 2025 to 2034. The North America dental bone grafts substitutes market size reached USD 347.12 million in 2024. Increasing the use of bone grafts in density and a growing number of dental implant surgeries are propelling the revenue.
Dental Bone Grafts Substitutes Market Key Takeaways
- The global dental bone grafts substitutes market was valued at USD 890.04 million in 2024.
- It is projected to reach USD 2,184.84 million by 2034.
- The dental bone grafts substitutes market is expected to grow at a CAGR of 9.40% from 2025 to 2034.
- North America led the market with the largest market share of 39% in 2024.
- Asia Pacific is projected to experience the highest CAGR of 11% during the forecast period.
- By material type, the xenograft product segment has accounted more than 49% of the market share in 2024.
- By material type, the synthetic segment is expected to grow at the fastest CAGR of 10.64% over the forecast period.
- By application, the socket preservation segment has generated more than 34% of market share in 2024.
- By application, the sinus lift segment is growing at a notable CAGR of 10.43% during the projected period.
- By end user, the dental clinic segment has held the largest market share of 77% in 2024.
- By end user, the hospital segment is projected to expand at the notable CAGR of 10.25% during the studied period.
U.S. Dental Bone Grafts Substitutes Market Size Size and Growth 2025 to 2034
The U.S. dental bone grafts substitutes market size was valued at USD 243.17 million in 2024 and is expected to be worth around USD 601.57 million by 2034, at a CAGR of 9.48% from 2025 to 2034.
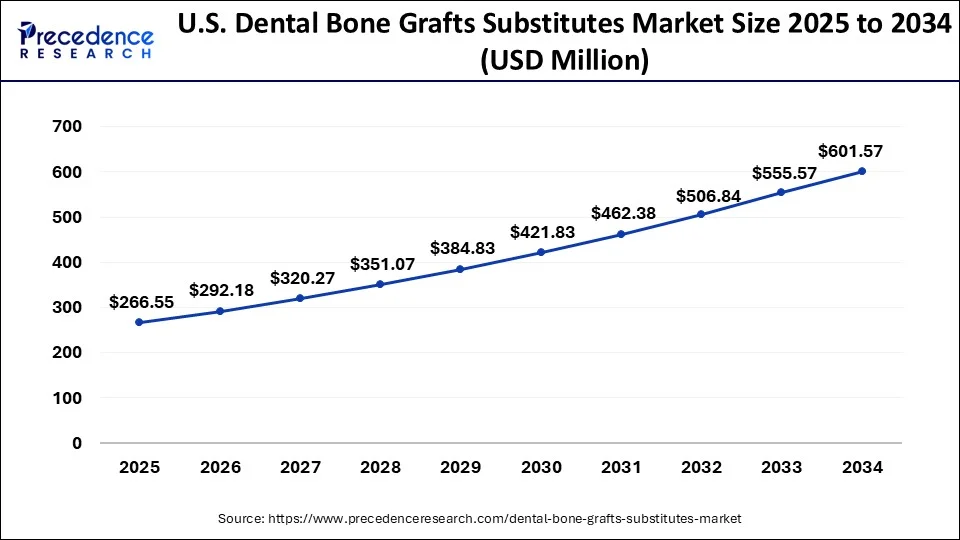
North America dominated the global dental bone grafts substitutes market in 2024. This was propelled by factors such as a growing population in need of dental implants and increasing awareness of advanced dental products. Moreover, factors like rising healthcare expenses and the accessibility of advanced healthcare facilities contributed to the region's growth. Additionally, the increasing prevalence of dental diseases played a significant role in driving market expansion.
- In January 2024, Dimension Inx CMFlex Pioneers Surgeries as FDA's First Cleared 3D Printed Bone Graft. Manufactured in December 2022, using the Desktop Health 3D-Bioplotter Manufacturer Series model, CMFlex has been successfully used in two patient jaw surgeries, an upper and lower jaw, and several dental socket preservation cases.
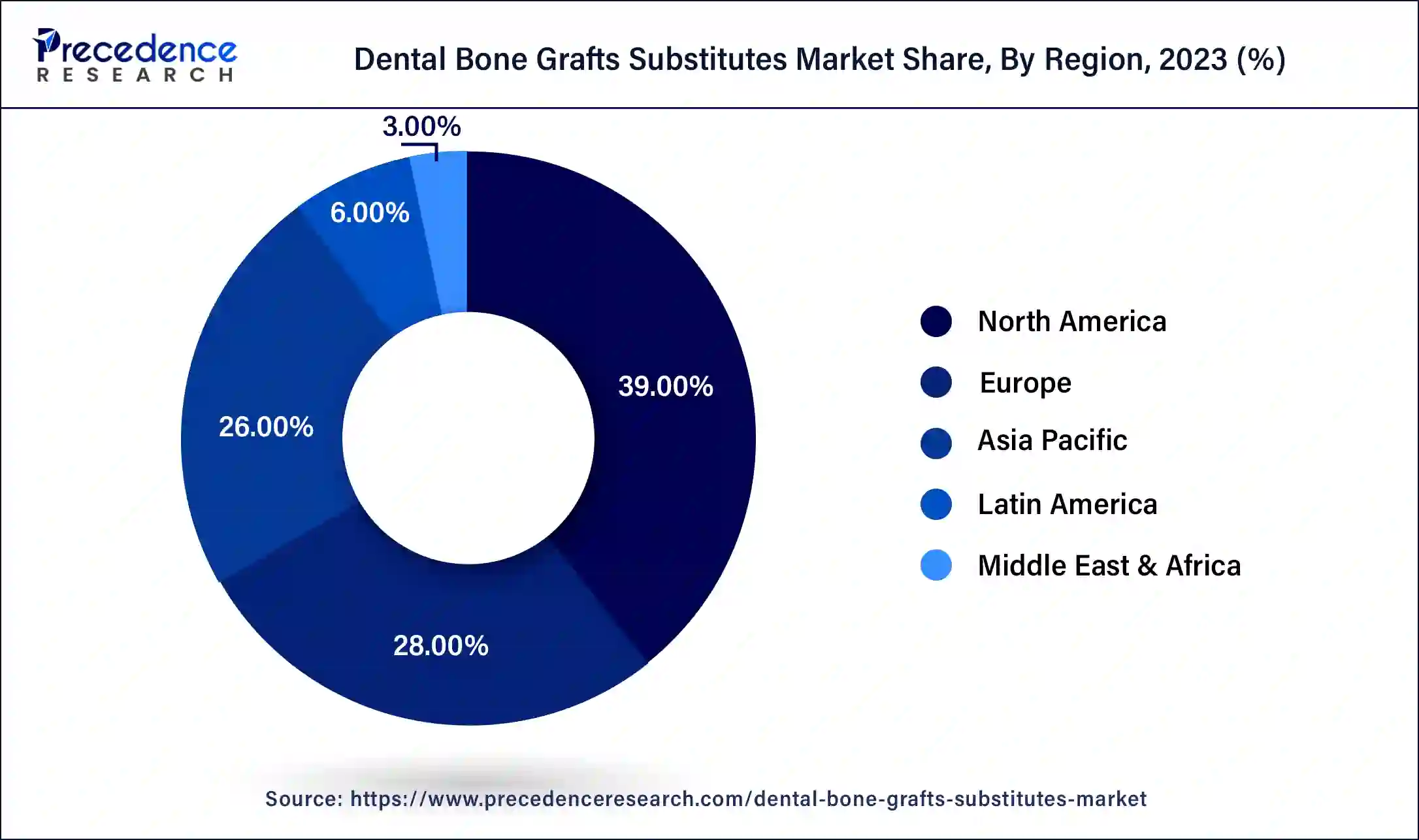
Asia Pacific is projected to experience the highest growth during the forecast period. This growth can be attributed to factors such as the increasing popularity of medical tourism and government initiatives. Furthermore, the region's aging population is contributing to a higher risk of dental issues. However, stringent regulatory guidelines in some countries may limit growth opportunities. In South Korea, products must be approved by the Korean Food and Drug Administration before being marketed, while in Australia, graft products are regulated by the Therapeutic Goods Administration. These strict regulations pose challenges for foreign players looking to enter the market.
Market Overview
Dental bone grafts create new bone that acts as a reservoir for minerals. Various substitutes for dental bone grafts include materials like bio-Oss, which is derived from bovine bone and like human bone, ceramic-based substitutes like calcium sulfate, and polymer-based substitutes like Healos. Synthetic bone grafts use materials like hydroxyapatite to mimic the mechanical properties of bone and facilitate bone growth for dental implants. Most studies use either allografts or xenografts as bone substitutes, which can be demineralized or mineralized freeze-dried bone allografts. Biomaterials used in dentistry include bone grafts and barrier membranes. The advancement of technology in biomaterials, driven by initiatives from key players and novel product launches, is expected to propel the dental bone grafts substitutes market.
Dental Bone Grafts Substitutes Market Growth Factors
- Rising success rate for dental surgeries can fuel the dental bone grafts substitutes market growth over the forecast period.
- The utilization of biocompatible & synthetic dental grafts can further propel the growth of the dental bone grafts substitutes market.
- Product launches by key market players can fuel the growth of the dental bone grafts substitutes market shortly.
- Growing dental tourism in emerging economies is one of the major factors contributing to the dental bone grafts substitutes market expansion.
- Technological advancement in the field of dentistry can drive the growth of market shortly.
Market Scope
| Report Coverage | Details |
| Growth Rate from 2025 to 2034 | CAGR of 9.40% |
| Market Size in 2024 | USD 890.04 Million |
| Market Size in 2025 | USD 974.86 Million |
| Market Size by 2034 | USD 2184.84 Million |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Material Type, Application, End-user, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Growing prevalence of dental disorders
Many oral health issues, like gum disease, tooth loss, tooth decay, and oral cancers, are largely preventable and can be treated, especially when addressed early in life. Factors such as inadequate fluoride exposure, easy access to sugary foods, and limited access to dental care contribute to these conditions. Moreover, conditions like orofacial clefts, Noma, and dental trauma are of significant public health concern. Consumption of sugary foods and drinks, tobacco and alcohol, further exacerbates oral health problems and other non-communicable diseases. This increasing prevalence of dental disorders is expected to drive the demand for the dental bone grafts substitutes market.
- In April 2023, orthobiologic products developer Bone Biologics received approval from the Human Research Ethics Committee in Australia to start the pilot study of its NB1 bone graft. Monash Health was approved as the first site to carry out a planned multi-center pilot clinical trial for the NB1 bone graft assessment.
Restraint
Expensive method of treatment
Bone grafts and substitute procedures can be costly, which may make them inaccessible to some patients, hence can limit the market growth. Certain healthcare systems have restrictive reimbursement policies for these procedures, making it challenging for patients, especially those with inadequate insurance coverage, to afford them. Furthermore, potential risks like infection, rejection, and nerve damage associated with these procedures also hinder the dental bone grafts substitutes market expansion.
Opportunities
Lucrative opportunities in developing countries
The dental bone grafts substitutes market is poised for growth, especially in emerging economies, driven by factors like improved healthcare infrastructure, rising demand for bone grafting procedures, and increasing prevalence of spinal disorders. Emerging economies are witnessing significant development in their healthcare sectors, fueled by government investments and the rise of medical tourism. This trend isn't limited to developed nations; countries like China, Brazil, and India are also experiencing a surge in demand for bone grafts and substitutes. Globally, there's a rising incidence of bone disorders such as osteoporosis and bone cancer, driving the need for these treatments to alleviate associated symptoms, which can create market opportunities in the future.
- In January 2023, Zimmer Biomet Holdings, Inc., a global medical technology leader, announced that it had reached a definitive agreement to acquire Embody, Inc., a privately held medical device company focused on soft tissue healing.
Material Type Insights
The xenograft product segment dominated the dental bone grafts substitutes market in 2024. Xenografts, sourced from pigs or cows, undergo sterilization and meticulous preparation before being implanted into humans. In dentistry, deproteinized bovine bone is commonly used for xenograft materials. Additionally, strategic initiatives by market players are contributing to the market's growth.
- In March 2022, Fredun Pharmaceuticals had permission to produce Xenografts for a range of dental and orthopedic surgical appliances from the Central Drugs Standard Control Organization (COSCO), India. The Company also plans to expand its reach within the dental community in India by doing this.
In the dental bone grafts substitutes market, the synthetic segment is expected to grow at the fastest rate over the forecast period. The growth of synthetic grafts can be credited to their superior osteoconductive, hardness, and higher acceptance rates. In the market, there are several types of synthetic materials available, including ceramic, polymer-based, and BMPs (bone morphogenetic proteins). Synthetic grafting carries a lower risk of disease transmission compared to xenografts and allografts, which further enhances its growth. There's a rising demand to develop biocompatible grafts to minimize adverse reactions, which is propelling this segment's expansion.
Application Insights
The socket preservation segment dominated the dental bone grafts substitutes market in 2024. This method aims to reduce bone loss that occurs after tooth extraction by filling the socket in the jawbone. The segment is growing due to the rising number of dental implant surgeries and increased awareness about oral health care among the population.
- In December 2023, a Madras varsity professor developed a multipurpose first-aid dental kit. The Advanced bioactive material in the ‘socket fit hemostat' retains its shape. The material used not only protects the cavity post-extraction but also heals the bone and prevents infection.
In the dental bone grafts substitutes market, the sinus lift segment is the fastest-growing segment during the projected period. This market is growing because more people are undergoing dental implant surgeries and becoming more aware of the significance of oral health care. Moreover, there's a growing burden of dental diseases, and advancements in dental technology are further fueling the growth of this segment.
End-user Insights
The dental clinic segment dominated the dental bone grafts substitutes market in 2024. This segment's growth is driven by an increasing number of dental clinics and the growing occurrence of dental issues. Dental clinics are gaining popularity due to their convenience and the availability of skilled surgeons, leading to a rise in the number of dental graft surgeries performed annually.
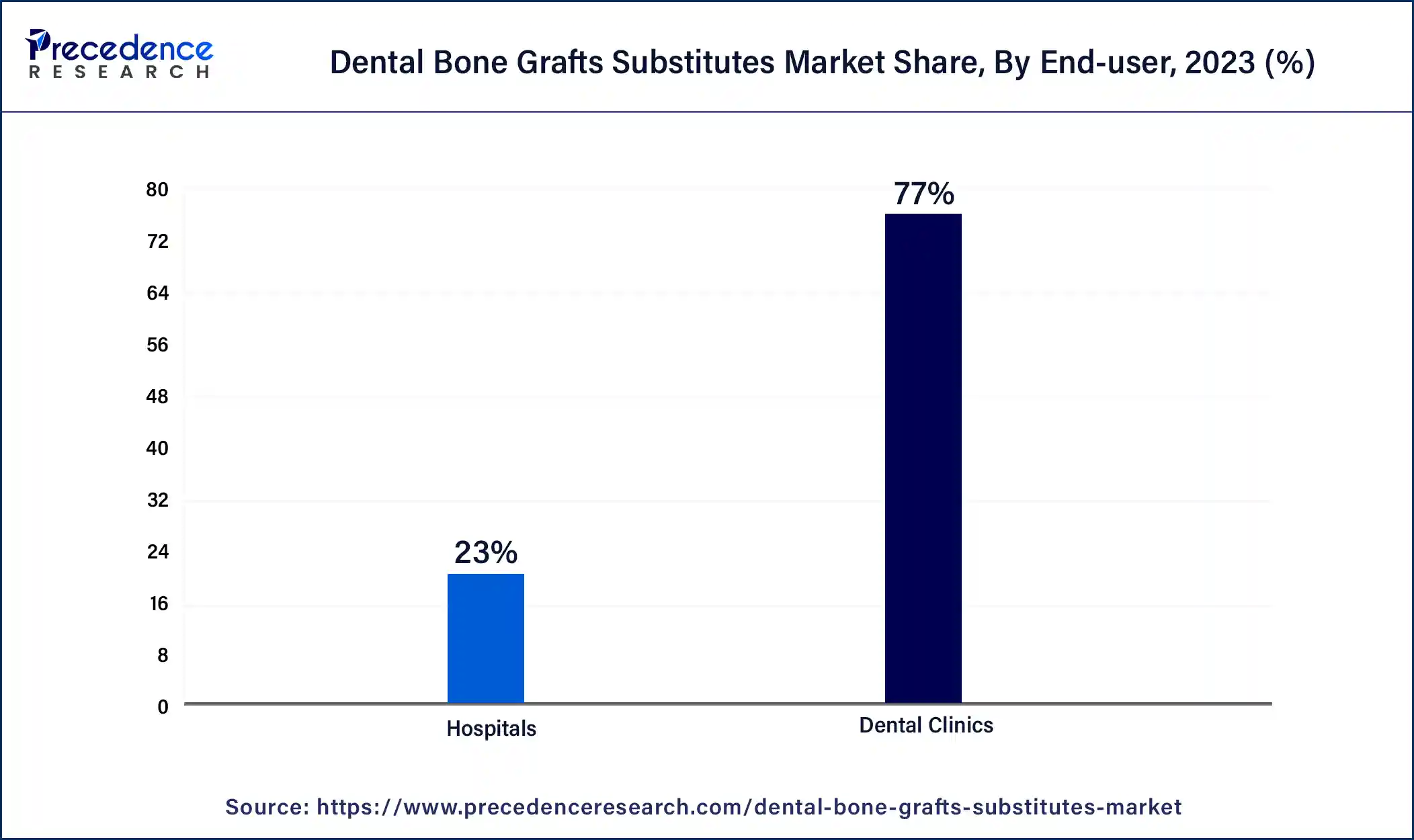
The hospital segment is the fastest-growing segment during the studied period. Hospitals see many patients, some of whom need orthopedic and dental procedures involving bone graft substitutes. This demand is significant due to the diverse medical services hospitals provide, including surgeries. Procedures like spinal fusion, joint replacements, and dental implants are commonly performed in hospitals, contributing substantially to the market for dental bone graft substitutes.
Dental Bone Grafts Substitutes Market Companies
- Medtronic (U.S.)
- Stryker Corporation (U.S.)
- Johnson & Johnson (U.S.)
- Zimmer Biomet Holdings, Inc. (U.S.)
- Baxter International Inc. (U.S.)
- NuVasive, Inc. (U.S.)
- Integra LifeSciences Corporation (U.S.)
- Wright Medical Group N.V. (Netherlands)
- Olympus Corporation (Japan)
- RTI Surgical Holdings, Inc. (U.S.)
- AlloSource (U.S.)
- Orthofix Medical Inc. (U.S.)
- Bioventus LLC (U.S.)
- SeaSpine Holdings Corporation (U.S.)
- Xtant Medical Holdings, Inc. (U.S.)
- Aziyo Biologics, Inc. (U.S.)
- Coloplast Corp. (Denmark)
- Arthrex, Inc. (U.S.)
- LifeNet Health (U.S.)
- RTI Surgical Holdings, Inc. (U.S.)
Recent Developments
- In May 2023, Zimmer Biomet Holdings, Inc. announced the launch of its VIVIX 3D Printed Bone Matrix, a breakthrough bone graft substitute with a porous structure designed to mimic the natural bone environment.
- In April 2023, Stryker Corporation acquired NuVasive, Inc., a leading provider of spine surgery solutions, expanding its product portfolio in the bone graft substitutes market and strengthening its presence in the spine care segment.
- In February 2023, Osseus Fusion Systems received FDA clearance for its ARIES-TS spinal implant system, designed for use with autograft or bone graft substitutes in posterior lumbar interbody fusion (PLIF) surgeries.
- In December 2022, Kuros Biosciences announced a collaboration with Johnson & Johnson Medical Devices Companies to co-develop innovative orthobiologic solutions for bone graft substitutes and regenerative medicine.
- In March 2022,A pioneer in regenerative medicine polymer technology, Molecular Matrix, Inc. (MMI), announced the full release of its Osteo-P Synthetic Bone Graft Substitute (BGS) for use in the musculoskeletal system.
- In September 2022,SeaSpine Holdings Corporation launched the Mariner MIS Posterior Fixation System, an innovative platform that combines pedicle screw fixation and bone grafting options for minimally invasive spinal fusion procedures.
Segments Covered in the Report
By Material Type
- Autograft
- Allograft
- Demineralized Bone Matrix
- Others
- Xenograft
- Synthetic
By Application
- Ridge Augmentation
- Sinus lift
- Periodontal Defect Regeneration
- Implant Bone Regeneration
- Socket Preservation
By End-user
- Hospitals
- Dental Clinics
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content