What is the Hepatitis D Market Size?
The global hepatitis D market size is calculated at USD 776.10 million in 2025 and is predicted to increase from USD 803.27 million in 2026 to approximately USD 1,092.71 million by 2035, expanding at a CAGR of 3.48% from 2026 to 2035.
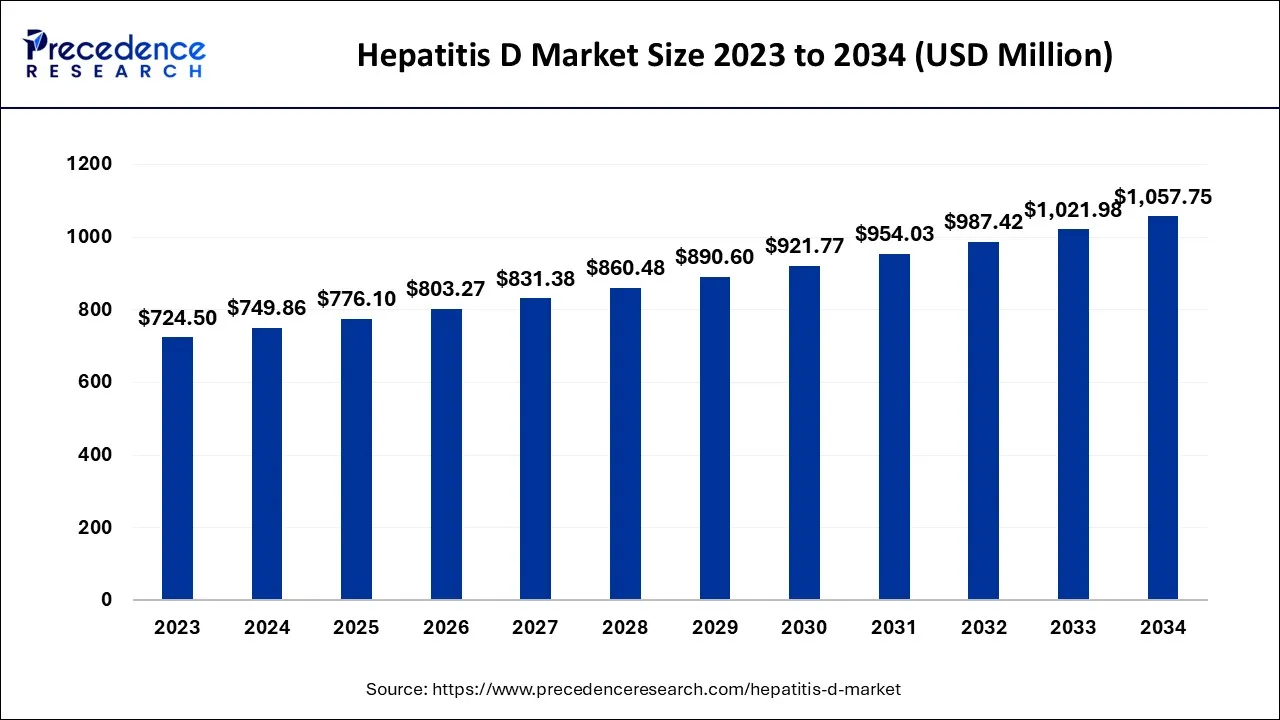
Hepatitis D Market Key Takeaways
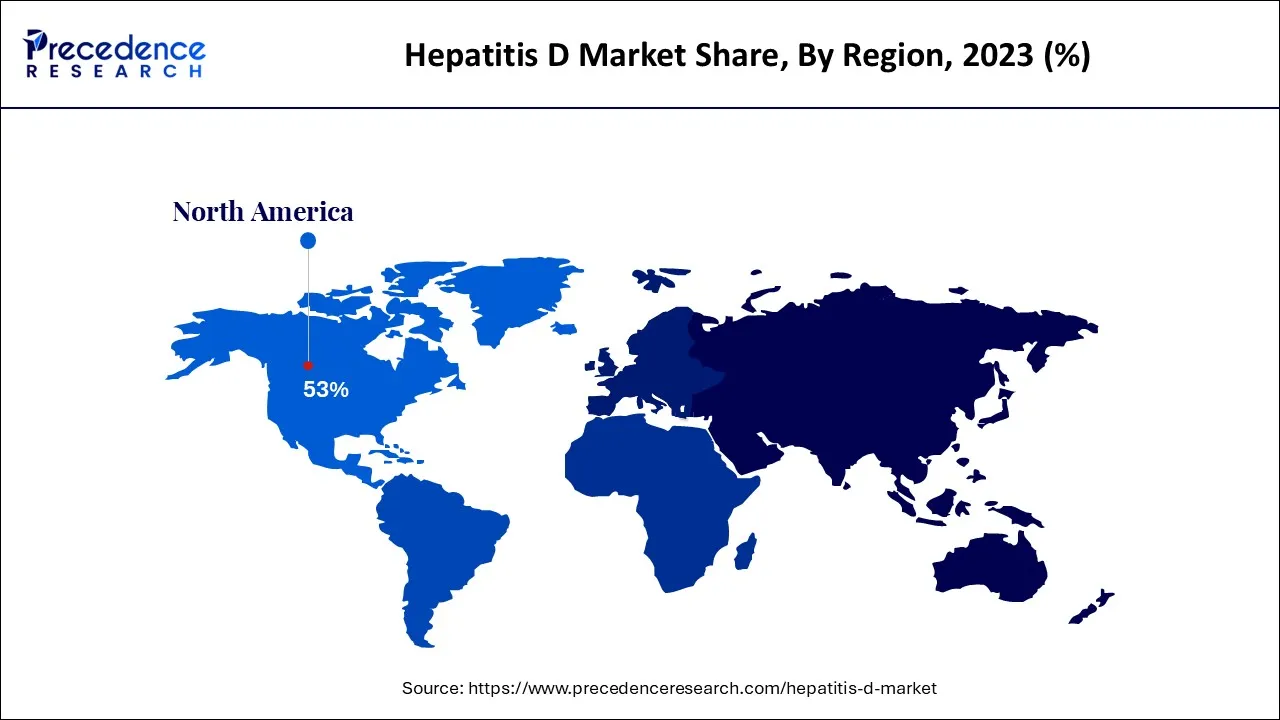
- North America led the global market with the highest market share of 53% in 2025.
- Asia Pacific is expected to expand at the fastest CAGR through 2025.
- By Type, chronic hepatitis segment contributed the largest share in 2025.
- By Distribution Channel, the hospital & retail pharmacies segment contributed the largest revenue share in 2025.
Strategic Overview of the Global Hepatitis D Industry
The hepatitis D virus (HDV), which depends on HBV for reproduction, causes hepatitis D, an inflammation of the liver. Absence of the hepatitis B virus prevents hepatitis D transmission. The co-infection of HDV and HBV is regarded as the most severe type of chronic viral hepatitis because it leads to hepatocellular carcinoma and liver-related mortality more quickly. The only way to avoid contracting HDV is throughhepatitis Bvaccination.
Artificial Intelligence: The Next Growth Catalyst in Hepatitis D
Artificial Intelligence is fundamentally transforming the hepatitis D industry by accelerating crucial processes from drug discovery to patient management. Machine learning algorithms analyze vast genomic and clinical datasets to identify novel antiviral targets and predict patient responses to therapies with high accuracy, significantly reducing R&D time and costs. In clinical practice, AI-powered diagnostic tools and advanced image analysis of MRIs and CT scans are improving the early detection of liver fibrosis and hepatocellular carcinoma, minimizing the need for invasive biopsies.
Furthermore, AI-driven platforms facilitate personalized medicine by tailoring treatment regimens to individual patient characteristics and using predictive models to track adherence and identify potential drug resistance.
Hepatitis D Market Growth Factors
The prevalence of hepatitis D and B is increasing, and eating an unbalanced diet frequently will slow the market's growth. Due to increasing healthcare costs, increased government funding, and increased efforts by both public and private groups to spread awareness of the disease, there will be an increase in demand for infections with the hepatitis delta virus (HDV). The market will continue to grow as a result of additional variables like alcohol abuse and the increasing prevalence of intravenous drug use. Additionally, concurrent HBV and HDV infection can cause a mild-to-severe or even fulminant hepatitis, whereas super-infection with HDV on chronic hepatitis B speeds up the progression of the disease in all ages and affects 70% to 90% of individuals.
- Alcohol addiction and an increase in the use of intravenous drugs
- An additional drug clearance
- New treatments are being developed
- Increasing prevalence of viral Hepatitis D
- Government support that is favorable for raising awareness of hepatitis
- Increasing incidence of chronic illnesses
- R&D in the pharmaceutical business is expanding
Market Outlook
- Market Growth Overview: The hepatitis D market is expected to grow significantly between 2025 and 2034, driven by the increasing awareness and diagnosis rates, advancement in therapeutics, and high prevalence of co-infection with hepatitis B.
- Sustainability Trends: Sustainability trends involve the increasing screening efficiency, cold chain optimization for biologics, and transition to non-invasive monitoring.
- Major Investors: Major investors in the market include Gilead Sciences, GSK, Johnson & Johnson, ARCH Venture Partners & OrbiMed, and F-Prime Capital.
- Startup Economy: The startup economy is focused on RNA-based therapeutics, novel entry inhibitors, and immunomodulators & vaccines as a cure.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 776.10 Million |
| Market Size in 2026 | USD 803.27 Million |
| Market Size by 2035 | USD 1,092.71 Million |
| Growth Rate from 2026 to 2035 | CAGR of 3.48% |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | By Type, By Diagnosis, and By Distribution Channel |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Key Market Drivers
Rise in the prevalence of hepatitis D
The defective single-stranded RNA virus known as hepatitis delta virus (HDV) depends on the presence of the hepatitis B virus (HBV) for both expression and replication. The viral infection hepatitis D results in liver inflammation. This further impairs liver functions and results in chronic liver issues like liver cancer and scarring.
In addition, hepatitis D signs include nausea, vomiting, fatigue, lack of appetite, jaundice, joint pain, abdominal pain, and dark urine. Now a days, hepatitis D prevalence is on the rise, and a high intake of an unbalanced diet will affect the market's growth rate. For instance, the Hepatitis D virus (HDV) affects nearly 5% of people worldwide who have a chronic Hepatitis B virus infection, according to data released by the WHO in 2022. (HBV).
Furthermore, the market for hepatitis delta virus (HDV) infections will grow as a result of rising healthcare costs, rising government funding, and rising government and private organization initiatives to raise awareness of the disease. In addition to this, other factors including alcohol abuse and increasing incidence of intravenous drug abuse will drive the market growth.
Research and development efforts have advanced
The rise in the prevalence of Hepatitis D increased R&D efforts, clinical trials of hepatitis drugs, an increase in the number of product launches and item endorsements, and the development of new hepatitis drugs for the treatment of various hepatitis types are the main factors driving the growth of the hepatitis therapeutics market.
The market is growing rapidly due to increasing collaborative research activities for the pipeline development of effective drugs. In addition, continued awareness of hepatitis treatment due to collaborations and associations in major organizations and the presentation of less approach to hepatitis treatment are other factors driving the market growth.
Government programs to raise knowledge of hepatitis and how to treat it
Hepatitis D is extremely prevalent and can cause serious liver cirrhosis, which can be fatal. Furthermore, it is anticipated that over the course of the forecast period, government initiatives to raise awareness about hepatitis and its treatment will spur market development. For instance, a virtual gathering called the "World Hepatitis Summit (WHS)" took place in June 2022.
The World Health Organization co-sponsored the World Hepatitis Summit 2022. (WHO). The summit offers a venue for the large hepatitis community to assess current development and exchange thoughts, stories, and best practices for overcoming the many difficulties associated with viral hepatitis.
Key Market Challenges
The cost of hepatitis treatments is high
However, the high cost of treatment and side effects from hepatitis D like liver failure, liver cirrhosis, and hepatocellular carcinoma will limit market expansion. The hepatitis delta virus (HDV) infection market will face additional challenges during the forecast period due to a lack of knowledge about preventative measures.
Furthermore, the hepatitis medications is predicted to grow slowly over the anticipated period due to the high capital needed for hepatitis pharmaceutical production. Because they are made from expensive basic materials like active pharmaceutical ingredients (APIs) and pharmacological intermediates, hepatitis medications are expensive. Since a skilled labor is needed, the intricate process of producing, separating, and utilizing raw materials for the creation of pharmaceutical and biopharmaceutical treatments raises the price of medications overall.
Key Market Opportunities
Rising ongoing clinical trials
Pegylated interferon is the only medication for hepatitis D that is currently authorised. (PEG-IFN). It works by boosting the immune system's ability to combat the infection. When administered once a week for 48 weeks, a tiny proportion of patients (30%) go into remission. New study indicates longer-term injections have higher success rates. Although they have no impact on hepatitis D, oral nucleosides (antivirals) approved for hepatitis B are occasionally used in conjunction with interferon therapy to help control high hepatitis B viral loads. In addition, seven medications are currently undergoing clinical studies to determine how well they work to treat the hepatitis D virus. (HDV). Therefore, an increase in clinical trials will also present market possibilities.
Segment Insights
Type Insights
Hepatitis D is further classified into chronic and acute. In 2025, the segment of chronic hepatitis had the largest proportion. In addition, new hepatitis drugs are being developed for the treatment of different hepatitis types, along with increased research and development efforts and hepatitis drug clinical trials.
Expanding study collaboration for the development of potent medications in the pipeline also encourages significant market growth. A unique factor that encourages market development is the promotion of affordable hepatitis therapeutics. This knowledge is being raised through alliances and joint efforts within significant organizations.
Diagnosis Insights
On the basis of diagnosis the market is categorized into blood tests, elastography, liver biopsy, serologic testing and others. In order to identify hepatitis D, doctors primarily use blood tests. High levels of anti-HDV immunoglobulin G (IgG) and immunoglobulin M (IgM) are also used to identify and confirm HDV infection, and HDV RNA can be found in serum. HDV RNA assays, which are used to assess response to antiviral therapy, are not standardized, and HDV diagnostics are not generally accessible.
Distribution Channel Insights
In 2025, the segment of hospital & retail pharmacies had the largest revenue share. The hospital & retail pharmacies sector is predicted to have the highest income in 2025 and to dominate the market throughout the projection period. The significant market share can be attributed to the numerous patients who seek Hepatitis D diagnosis and treatment immediately at the hospital. In addition, the sector is anticipated to have significant growth opportunities due to the rising number of hospital & retail pharmacies providing Hepatitis D treatments in emerging economies.
Regional Insights
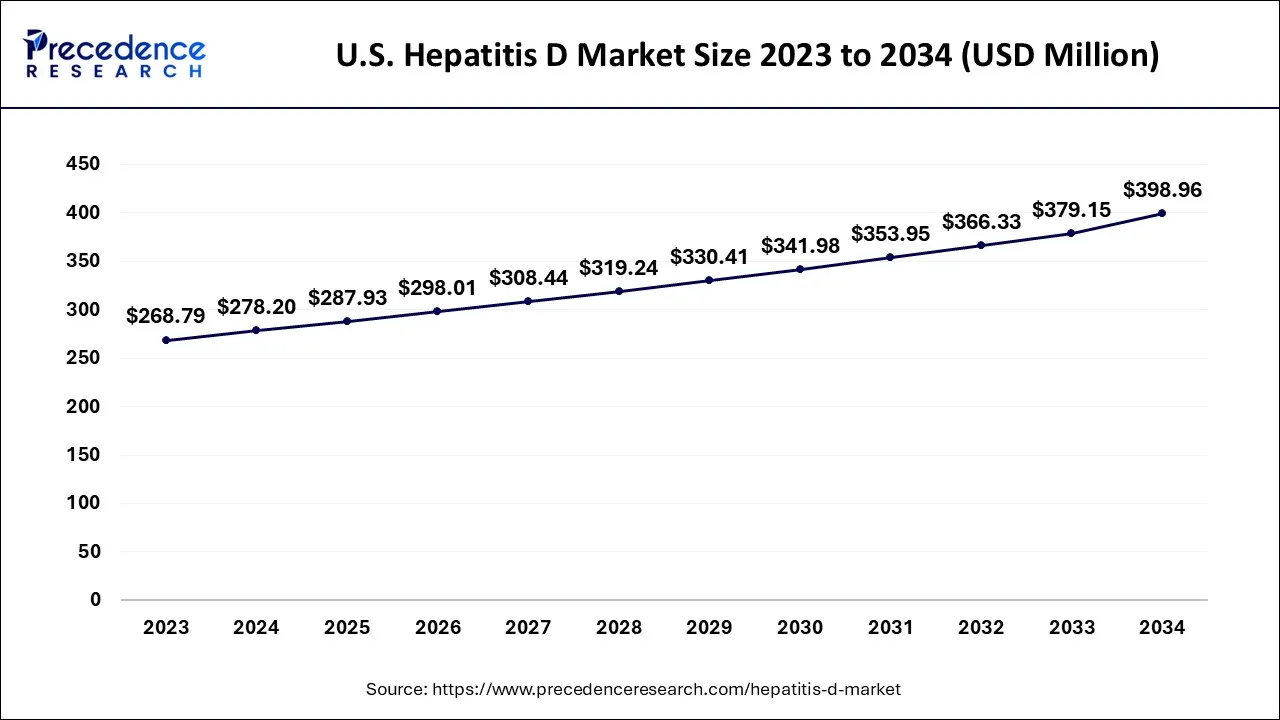
U.S. Hepatitis D Market Size and Growth 2026 to 2035
The U.S. hepatitis D market size is exhibited at USD 287.93 million in 2025 and is projected to be worth around USD 414.11 million by 2035, growing at a CAGR of 3.70% from 2026 to 2035.
Why Did North America Lead the Global Hepatitis D Market in 2025?
North America led the Hepatitis D market by capturing the largest share in 2025. This is mainly due to its advanced healthcare infrastructure and widespread availability of diagnostic and treatment facilities. High awareness of hepatitis co-infections and proactive screening programs have enabled early detection and effective disease management. The region benefits from substantial healthcare spending and strong clinical research activity, particularly in the U.S., which drives the development and adoption of novel therapies. Favorable reimbursement policies and the presence of specialized hepatology centers further support market growth and patient access to care
U.S. Hepatitis D Market Trends
The U. S's robust pipeline of novel treatments like bulevirtide and advancements in molecular diagnostics and non-invasive testing for earlier detection. Strong public health initiatives are expanding integrated screening for high-risk populations, primarily intravenous drug users, fostering proactive disease management.
Why is Asia Pacific Considered the Fastest-Growing Region in the Hepatitis D Market?
Asia Pacific is expected to grow at the highest CAGR in the market because of the prevalence of chronic hepatitis B, which directly puts the population at risk of co-infection with HDV. The detection rates of HDV are increasing due to improved healthcare access, the expansion of viral hepatitis screening programs, and increased investments in the management of infectious diseases. Governments and health agencies are placing greater emphasis on viral hepatitis elimination programs, thereby supporting long-term demand for HDV therapies. Additionally, global pharmaceutical corporations are expanding their presence by entering into regional agreements, conducting local clinical trials, and implementing tiered pricing to enhance affordability.
India Hepatitis D Market Trend
The National Viral Hepatitis Control Program (NVHCP) remains the primary market driver, offering free diagnostics and treatment to support the goal of viral elimination by 2030. India's strengths in generic manufacturing ensure high affordability for antiviral medications, while advanced diagnostics and non-invasive elastography are becoming standard in hospital-based care. Although specific HDV therapies remain limited, ongoing global clinical trials for RNA-based treatments are expected to expand future local options.
What Makes Europe a Notably Growing Area in the Hepatitis D Market?
Europe is expected to experience notable growth in the coming years, driven by strong clinical awareness, early adoption of approved HDV therapies, and well-organized healthcare systems that support rare disease treatment. Emphasis on evidence-based medicine, centralized hepatology centers, and extensive patient registries enhances disease management and treatment outcomes. Countries like Germany, France, and Italy contribute to demand through high-quality diagnostics and access to specialized treatments supported by insurance coverage. Additionally, EU-level strategies aimed at eradicating viral hepatitis further promote early detection and treatment, boosting overall market development.
How Did Europe Notably Grow in the Hepatitis D Market?
The European hepatitis D market is expanding through its global first-mover advantage, following the EMA's pioneering full approval of HDV-specific therapies such as bulevirtide. Growth is sustained by standardized "reflex testing" protocols and specialized liver centers that efficiently manage high-prevalence migrant and high-risk populations. This regulatory maturity, combined with a robust clinical trial ecosystem, has established Europe as the primary hub for advanced antiviral adoption and innovative diagnostic integration.
Germany Hepatitis D Market Trend
Germany's early adoption of the world's first EMA-approved HDV therapies, a systematic "reflex testing" protocol for all Hepatitis B patients, and high public health awareness campaigns. With a large network of specialized liver centers and strong insurance reimbursement, the market is characterized by high diagnostic rates and rapid integration of novel entry inhibitor treatments.
Why is the MEA Hepatitis D Market Gaining Momentum?
The Middle East & Africa (MEA) Hepatitis D market is growing due to improved healthcare facilities and increased awareness of viral hepatitis and screening programs in the region. The Middle East has a high incidence of HBV and HDV co-infections, particularly among migrants and high-risk groups. Access to diagnosis and treatment is increasing through government funding for infectious disease control, the establishment of specialty liver clinics, and collaboration with pharmaceutical firms worldwide. As knowledge grows and new treatments become available, the MEA region has long-term growth potential for HDV market players.
What Potentiates the Market in Latin America?
The Hepatitis D market in Latin America is driven by increased awareness, enhanced disease surveillance, and growing attention to hepatitis viruses. Countries such as Brazil, Argentina, and Peru are strengthening HBV and HDV monitoring systems, particularly in endemic and underserved regions. Healthcare systems are gradually adopting more advanced diagnostics and specialized liver treatments, improving patient identification rates. Market expansion is further supported by participation in international clinical trials and collaborations with global pharmaceutical companies seeking growth in the region.
Value Chain Analysis of the Hepatitis D Market
Drug Discovery & Advanced Research (R&D): This foundational stage focuses on identifying novel viral targets such as the HBsAg entry mechanism or farnesyltransferase inhibition.
- Key Players: Gilead Sciences, Vir Biotechnology, Alnylam Pharmaceuticals, and Assembly Biosciences.
Specialized Diagnostic Development:This stage involves the manufacturing of high-sensitivity molecular assays, such as HDV-RNA PCR tests and nucleic acid testing (NAT) platforms
- Key Players:Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and Hologic.
API & Pharmaceutical Manufacturing:Manufacturing in the HDV market involves producing complex biologicals, such as entry inhibitors like Bulevirtide, and traditional Pegylated Interferons.
- Key Players:Lonza Group (Biologics), Zydus Lifesciences, Hetero Labs, and Catalent.
Specialized Distribution & Cold Chain Logistics:Many emerging HDV therapies are biologics that require strict temperature-controlled environments to maintain stability.
- Key Players: Cryoport, DHL Life Sciences, AmerisourceBergen, and Cardinal Health.
Clinical Delivery & Specialist Care:The final value stage occurs in specialized hepatology clinics and transplant centers where treatment is administered and monitored via non-invasive elastography.
- Key Sectors: Academic Medical Centers, Hepatology Clinics, Specialty Pharmacies, and Public Health Agencies (e.g., CDC, NVHCP).
R&D: Pharmaceutical and biotechnological firms are focusing on identifying new antiviral activities and entry inhibitors that target the replication of the Hepatitis D virus and co-infection with HBV.
- Key players: Gilead Sciences, Arrowhead Pharmaceuticals, Eiger BioPharmaceuticals, Janssen Pharmaceuticals
Clinical Trials and Regulatory Approvals: Multi-phase clinical trials are conducted on drug candidates, with regulatory review to establish safety, efficacy, and long-term virologic response in HDV patients.
- Key players: Eiger BioPharmaceuticals, Gilead Sciences, Roche, FDA / EMA
Formulation and Final Dosage Preparation:Approved HDV therapeutic agents are developed into injectable or oral formulations with enhanced stability, bioavailability, and patient compliance.
- Key players:Lonza Group, Catalent, Thermo Fisher Scientific, Patheon (Thermo Fisher)
Top Companies in the Hepatitis D Market & Their Offerings
- Biosidus: Biosidus contributes to the Hepatitis D market as a key regional manufacturer of biosimilar interferons, providing affordable treatment options in emerging markets like Latin America.
- F. Hoffmann-La Roche Ltd:Roche is a leader in the diagnostic segment, providing high-sensitivity molecular assays and PCR platforms essential for the accurate detection of HDV-RNA levels.
- NanoGen Healthcare Pvt. Ltd.:NanoGen focuses on the development and distribution of high-quality biopharmaceuticals, including interferon therapies tailored for the Asian market. They play a significant role in improving the accessibility of viral hepatitis treatments through localized manufacturing and strategic healthcare partnerships.
- AMEGA Biotech:AMEGA Biotech specializes in the large-scale production of recombinant proteins and biosimilar interferons used in the treatment of chronic viral hepatitis. They strengthen the global supply chain by offering cost-effective alternatives to branded biologics, particularly in developing regions.
- Rhein-Minapharm:Rhein-Minapharm leverages its expertise in bioprocess engineering to manufacture high-complexity biologics, including interferon-based therapies for the Middle East and Africa. Their contributions are vital for managing the high prevalence of Hepatitis D in endemic regions through localized, high-standard production.
- PROBIOMED SA de CV:PROBIOMED is a major Mexican biopharmaceutical firm that produces biosimilar interferons used to treat various viral infections, including Hepatitis D.
- 3SBio Group:3SBio is a Chinese biotechnology leader that produces interferon-based treatments and focuses on expanding clinical research for liver diseases.
- Eiger BioPharmaceuticals:Eiger is at the forefront of HDV research with its lead candidate Lonafarnib, a first-in-class farnesyltransferase inhibitor designed to block the assembly of the virus. They are pivotal in advancing specialized, targeted therapies through global Phase III clinical trials to address high unmet medical needs.
- Hepion Pharmaceuticals, Inc.:Hepion is developing Rencofilstat, a cyclophilin inhibitor that targets the underlying mechanisms of liver injury and viral replication in HDV and NASH. Their contribution lies in advancing unique pleiotropic drug candidates that address both the viral load and the associated liver fibrosis.
- Antios Therapeutics, Inc.:Antios focuses on developing ATI-2173, a novel Active Site Polymerase Inhibitor Nucleotide (ASPIN) designed to provide a potentially curative treatment for chronic Hepatitis B and D.
- PharmaEssentia Corporation:PharmaEssentia contributes through its development of Ropeginterferon alfa-2b, a long-acting monopegylated interferon with improved tolerability profiles. This next-generation interferon provides a more convenient dosing schedule for patients requiring long-term treatment for chronic viral hepatitis.
- Replicor:Replicor is pioneering Nucleic Acid Polymers (NAPs) that aim to clear the HBsAg from the blood, a critical step for achieving a functional cure in HBV/HDV co-infected patients.
- Janssen Pharmaceuticals, Inc.:Janssen, a subsidiary of Johnson & Johnson, invests in diverse portfolios of RNA interference (RNAi) and entry inhibitors targeting the Hepatitis B and D viral cycles.
- Apotex Corp.:Apotex is a major generic manufacturer that improves market competition by providing low-cost versions of supportive medications used in liver disease management. Their broad distribution network ensures that patients have access to essential components of the broader antiviral treatment stack.
- Mylan N.V. (Viatris):Mylan contributes by producing high-volume generic antiviral therapies and supportive care drugs through its extensive global manufacturing footprint. They play a critical role in large-scale public health programs by making chronic hepatitis treatments more affordable for government-led initiatives.
- Aurobindo Pharma Limited:Aurobindo is a key global player in the manufacturing of cost-effective antiviral APIs and finished dosages, particularly for markets with a high burden of Hepatitis B.
- Gilead Sciences, Inc.:Gilead is the global market leader in Hepatitis D, holding the approval for Hepcludex (Bulevirtide), the first-in-class entry inhibitor for chronic HDV.
- GlaxoSmithKline (GSK):GSK is advancing Bepirovirsen, an antisense oligonucleotide designed to reduce viral proteins and stimulate the immune system to fight Hepatitis B and D.
- Zydus Pharmaceuticals:Zydus contributes to the market through the development of biosimilars and high-quality generic antivirals, supporting the National Viral Hepatitis Control Program in India.
Recent Developments
- In December 2025, Bluejay Therapeutics, a biotechnology company developing a cure for hepatitis D, was acquired by Mirum Pharmaceuticals, a rare disease drugmaker, in a transaction valued at up to USD 820 million. The acquisition enhances Mirum's pipeline for rare liver diseases and paves the way for HDV therapeutics. (Source: pharmatimes.com)
- In April 2025, the first Integrated Development and Manufacturing Organization (IDMO), Cellares, received the first-ever FDA Advanced Manufacturing Technology (AMT) designation for its Cell Shuttle, allowing for expedited priority review of therapies manufactured on the platform. This technology is reflecting the Cell Shuttle's ability for automating and reliably manufacture cell therapies (Source: cellares.com)
- In December 2024, nChroma Bio was formed through the merger of Chroma Medicine and Nvelop Therapeutics, combining epigenetic editing capabilities with non-viral in vivo delivery platforms. The company completed an oversubscribed USD 75 million financing round to advance its lead candidate, CRMA-1001, a liver-directed epigenetic editing treatment for the potential functional treatment of chronic HBV and HDV. (Source: contagionlive.com)
- In April 2022, an international biopharmaceutical firm called Antios Therapeutics, Inc. has created cutting-edge therapies for the management of chronic Hepatitis D virus. The U.S. Patent and Trademark Office recently revealed that the patent application for phosphoramidates for the treatment of Hepatitis D virus had been granted. Along with other treatments and processes, the compositions include the brand-new Active Site Polymerase Inhibitor Nucleotide (ATI-2173) from Antios.
- In January 2022, Tenofovir alafenamide tablets manufactured by Lupin were given the go-ahead by the US Food and Drug Administration to treat chronic Hepatitis D viral infection.
Segments Covered in the Report
By Type
- Acute
- Chronic
By Diagnosis
- Blood Tests
- Elastography
- Liver Biopsy
- Serologic Testing
- Others
By Distribution Channel
- Hospital & Retail Pharmacies
- Online Pharmacies
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content



