What is Point-of-Care Diagnostics Market Size?
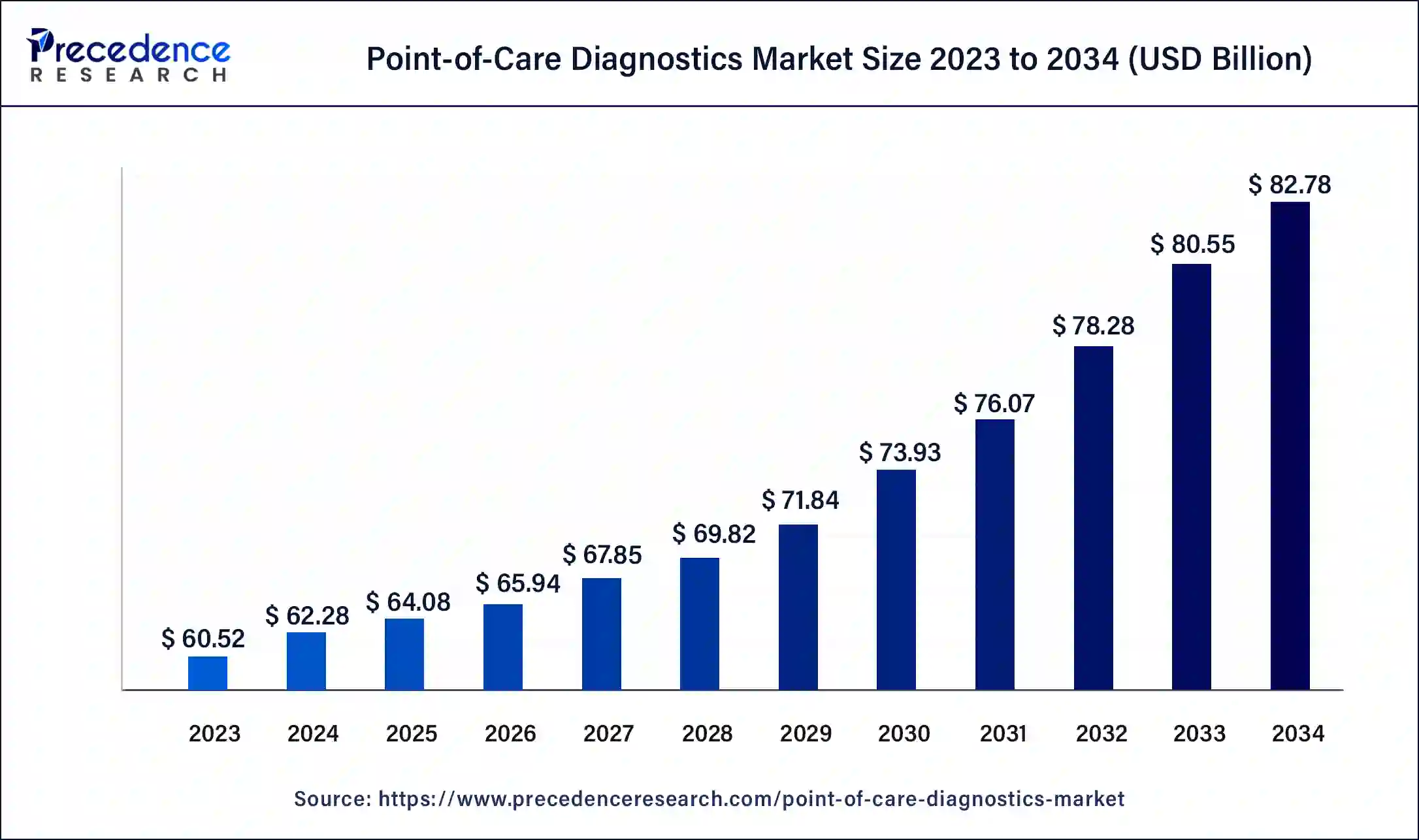
The global point-of-care diagnostics market size accounted for USD 64.08 billion in 2025 and is predicted to reach around USD 85.03 billion by 2035, growing at a CAGR of 2.87% from 2026 to 2035. The point of care diagnostics market is driven by the rising incidence of chronic diseases, mainly diabetes.
Market Highlights
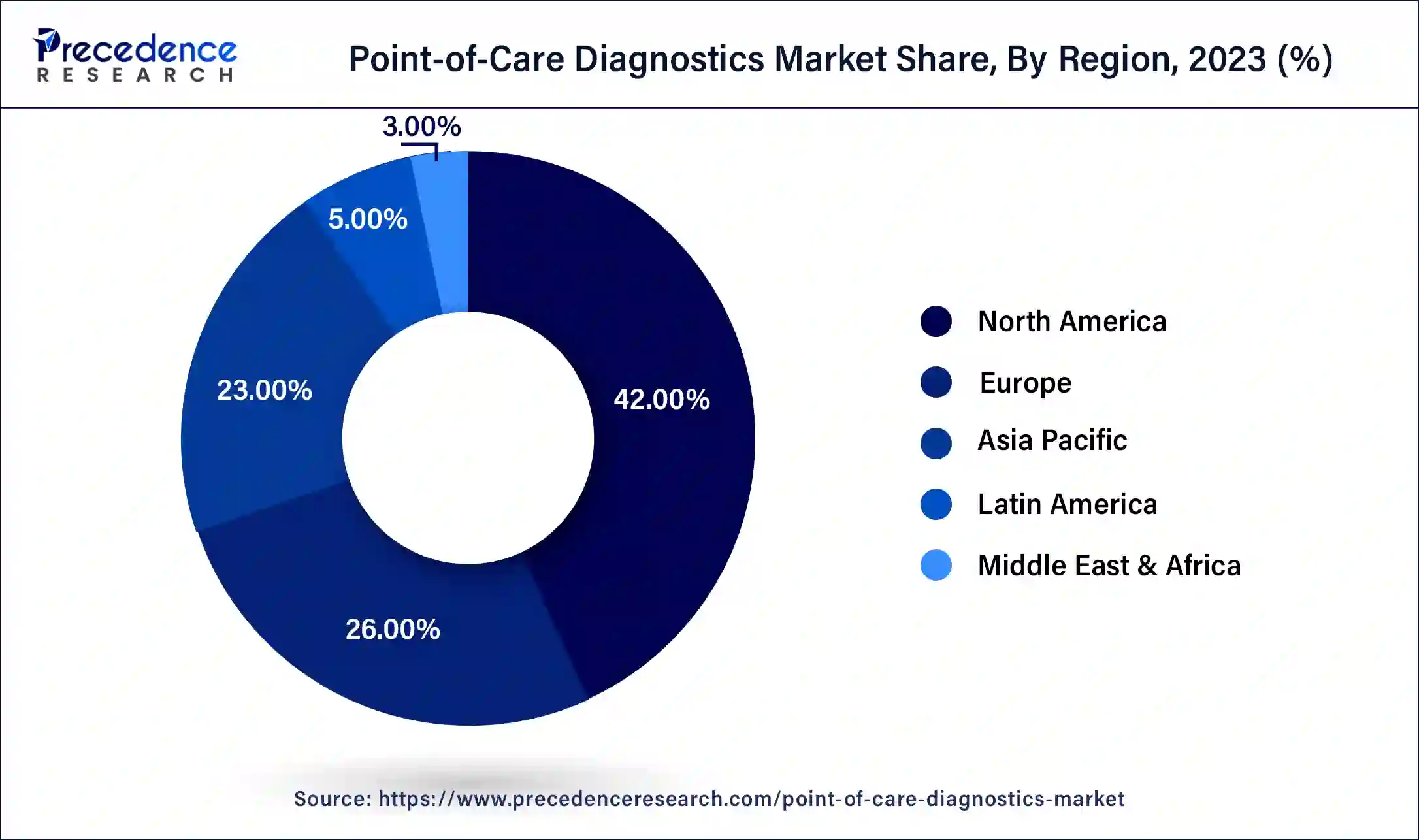
- The North America region accounted for 42% of the total revenue share in 2025.
- By product, the infectious diseases segment accounted for the highest market share 61% in 2025. The glucose testing segment has captured the second position for revenue holders in 2025.
- By end user, the clinics segment has captured a revenue share of around 38% in 2025.
- The home end-use segment is growing at a CAGR of 3.6% from 2026 to 2035
- The Asia Pacific is the fastest-growing segment in the near future.
Next-Gen Diagnostics: How AI is Transforming Point-of-Care Testing
Artificial intelligence is significantly improving point-of-care diagnostics by enhancing speed, accuracy, and accessibility of testing. AI algorithms can determine complex datasets, detect subtle patterns in medical images, and also integrate information from numerous sources such as lab tests, imaging, and patient histories, contributing to more precise and timely diagnoses. AI permits more tests to be done at the point of care, closer to individuals, mainly in remote or resource-limited settings. AI algorithms can detect individual patient information to tailor treatment plans and forecast potential future health risks.
Point of Care Diagnostics Market Overview
The medical devices utilized to get an instant result in the investigation (diagnosis & monitoring) of various diseases, such as cancer, diabetes, heart diseases, and others, are referred to as point-of-care (POC) diagnostics. The global point-of-care diagnostics industry has a lot of room for growth for both established and new companies. The point-of-care diagnostics market is growing due to technological developments in POC devices, rising infectious disease incidence, and increased expenditures by key companies. Point-of-care devices provide a much-needed boost in the direction of a patient-centric approach. As a result, the point-of-care diagnostics market may see a significant increase in growth rate during the forecast period.
Point-of-care diagnostics have changed patient care because of their simplicity and efficiency. POC technologies have evolved in efficiency. Point-of-care diagnostics (POC) technologies have evolved and improved as the area of microfluidics has progressed. Microfabrication and microfluidics methods have advanced so much in the last several years that POC devices can now be made at a cheap cost, are simple to use, portable, and produce quick results. In the subject of microfluidics technology, there are numerous research projects underway.
Due to the usage of microfluids, nano diagnostics, and chips, point-of-care diagnostics is gaining widespread acceptability among patients throughout the world. The samples are taken from the patient's site for completing tests, and findings can be obtained in a very short amount of time.
Point-of-Care Diagnostics Market Growth Factors
The medical devices utilized to get an instant result in the investigation (diagnosis & monitoring) of various diseases, such as cancer, diabetes, heart diseases, and others are referred to as point-of-care (POC) diagnostics in this report. The global point-of-care diagnostics industry has a lot of room for growth for both established and new companies. The point-of-care diagnostics market is growing due to technological developments in POC devices, rising infectious disease incidence, and increased expenditures by key companies.
The rising occurrences of target disorders, as well as the increased prevalence of infectious diseases such as tuberculosis and AIDS, are boosting the expansion of the point of care diagnostics industry. The growing desire for rapid tests among the general public is also helping the point-of-carediagnostics industry grow. The need to make the healthcare sector more patient-centered is proven to be a positive factor in the point-of-care diagnostics market's growth rate. Many patients may find the centralized testing method inconvenient because of it necessitates multiple trips to clinics to complete the entire evaluation process. Point of care devices provides a much-needed boost in the direction of a patient-centric approach. As a result, the point-of-care diagnostics market may see a significant increase in growth rate during the forecast period.
Point-of-care diagnostics has changed patient care because of their simplicity and efficiency. POC technologies have evolved efficiency. Point-of-care diagnostics (POC) technologies have evolved and improved as the area of microfluidics has progressed. Microfabrication and microfluidics methods have advanced so much in the last several years that POC devices can now be made at a cheap cost, are simple to use, portable, and produce quick results. In the subject of microfluidics technology, there are numerous research projects underway.
Due to the usage of micro fluids, nano diagnostics, chips, point-of-care diagnostics is gaining widespread acceptability among patients throughout the world. The samples are taken from the patient's site for completing tests, and findings can be obtained in a very short amount of time.
The increased frequency of chronic and infectious in developing economies is one of the primary factors driving the growth of point-of-carediagnostics market. The chronic diseases such as rheumatism, diabetes, and cancer are on the rise around the world for a variety of causes, including an ageing population, unhealthy lifestyle, and environmental factors.
The adoption of glucose monitoring testing kits is driven by factors such as the need for early detection of hyperglycemic and hypoglycemic diabetes, the high prevalence of diabetes, the convenience of continuous glucose monitoring over traditional monitoring, availability of glucose monitors, and increased awareness of the products around the world, and growing technological innovations.
Point-of-Care Diagnostics Market Outlook
- Industry Growth Overview:Between 2025 and 2034, the point-of-care diagnostics market is expected to grow rapidly due to rising demand for quick and near-patient testing solutions. This growth is driven by the increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and infectious diseases. Additionally, higher healthcare spending in emerging economies and growing awareness of the importance of early disease detection are supporting market expansion.
- Innovation & Technology Trends:Technological advancements are transforming the POC diagnostics landscape, with innovations in microfluidics, lab-on-a-chip systems, and molecular diagnostic platforms. Accuracy, speed, and remote monitoring of POC tests are being improved through AI-based analysis and cloud connectivity. Companies are focusing on multiplex testing, where multiple biomarkers can be detected on a single sample. POC solutions are becoming more user-friendly, accessible, and suitable for a wide range of clinical environments thanks to these innovations.
- Global Expansion:Market leaders are strategically expanding their geographic reach to meet increasing demand and take advantage of favorable regulatory environments. Companies like Siemens Healthineers, Thermo Fisher Scientific, and Roche are setting up hubs and service centers in the region to ensure quick delivery and technical support. The international expansion also helps these companies meet regulatory compliance and local quality standards, making market entry easier.
- Major Investors:Investment activity in the POC diagnostics industry is accelerating as it attracts attention from private equity, venture capital, and strategic investors, along with its growth potential. The technical barriers to entry are high, healthcare diagnostic resilience remains steady, and the sector aligns well with government health initiatives, making it appealing. The influence of major financial players is also boosting R&D projects and the commercialization of next-generation POC solutions.
- Startup Ecosystem:The POC diagnostics startup ecosystem is rapidly growing, driven by innovation, unmet clinical needs, and venture capital funding. New molecular testing platforms, AI-enabled diagnostics, and integrated digital healthcare solutions are emerging within startups to improve accessibility and efficiency. Collaborations with hospitals, research institutions, and pharmaceutical companies are helping these startups grow quickly. Additionally, product development is becoming more inclusive and globally impactful, emphasizing affordability, scalability, and sustainability.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 64.08 Billion |
| Market Size in 2026 | USD 65.94 Billion |
| Market Size by 2035 | USD 85.03 Billion |
| Growth Rate from 2026 to 2035 | CAGR of 2.87% |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Product, End User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising Tide of Chronic Disease in the United States
|
Year |
Prevalence of Chronic Disease (Millions of Americans) |
|
1995 |
118 |
|
2000 |
125 |
|
2005 |
133 |
|
2010 |
141 |
|
2015 |
149 |
|
2020 |
157 |
|
2025 |
164 |
|
2030 |
171 |
How is the increasing prevalence of chronic and infectious diseases the driver for the point-of-care diagnostics market?
The increasing prevalence of both chronic and infectious diseases is a major driver for the point-of-care diagnostics market. This is because point-of-care testing provides faster, more accessible, and usually more cost-effective diagnostic solutions, which are mainly beneficial for managing these conditions. Point-of-care molecular diagnostics provide faster turnaround times for infectious disease testing, vital for prompt diagnosis as well as treatment initiation, mainly for highly contagious diseases.
Restraint
How does complexity in regulations and reimbursement policies restrain the point-of-care diagnostics market?
Complexity in regulations and reimbursement policies significantly restrains the point-of-care diagnostics market by increasing costs, delaying market entry, and hindering widespread adoption. These factors disproportionately affect smaller firms and those in resource-restricted settings. Securing reimbursement for point-of-care tests, especially in community or home settings, can be challenging due to varying policies along with the preference for conventional testing methods. Shortage of clear reimbursement pathways can discourage acceptance, even when tests are available.
Opportunity
How are technological advancements an opportunity for the point-of-care diagnostics market?
Technological advancements are driving significant growth and opportunity within the point-of-care diagnostics market. Innovations such as miniaturization, biosensors, microfluidics, AI integration, and multiplexing capabilities are making point-of-care testing quicker, more accurate, and more accessible, contributing to increased adoption over various healthcare settings. Technological advancements are contributing to smaller, more portable point-of-care devices, permitting testing in diverse settings such as mobile healthcare units, remote clinics, and even at home.
Segment Insights
Product Insights
Based on the product, the infectious diseases segment dominated the market with the highest share 61% in 2025. Due to the increased prevalence of various infectious disorders, the infectious diseases segment of the market is expected to grow significantly during the forecast period.
On the other hand, the glucose testing segment has captured the second position in 2025. The rising diabetic and cancer population, the adoption of new point-of-care diagnostics technologies, and the growing preference for home glucose testing are all factors that contribute to the growth of the glucose testing segment.
End User Insights
Based on the end user, hospital bedside led the market with highest share in 2025. Due to the rising prevalence of chronic diseases requiring long term care and frequent monitoring in critical care units, as well as rapidly growing awareness of the availability of cost-effective and highly innovative point of care diagnostics products, this segment accounted for the largest share of the point-of-care diagnostics market.
On the other hand, urgent care and retail clinic segment is expected to grow at a faster rate during the forecast period. The increase in the number of clinics and urgent care centers, as well as the government's measures to improve patient care in hospitals through the use of quick point of care diagnostics tests and other programs, are expected to drive the expansion of this segment during the forecast period.
Regional Insights
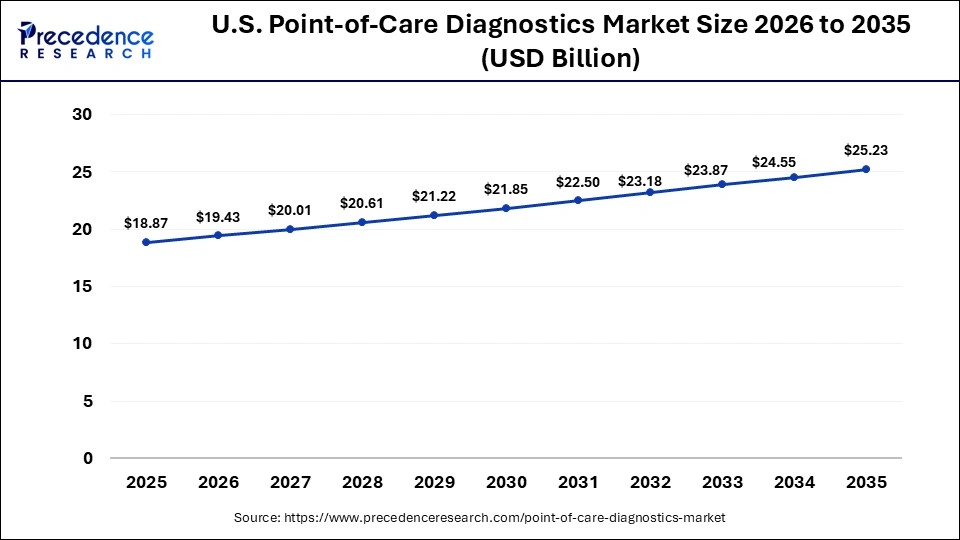
U.S. Point-of-Care Diagnostics Market Size and Growth 2026 to 2035
The U.S. point-of-care diagnostics market size is valued at USD 18.87 billion in 2025 and is expected to reach around USD 25.23 billion by 2035, growing at a CAGR of 2.95% from 2026 to 2035
U.S. Point-of-Care Diagnostics Market Analysis
The North American point-of-care diagnostics market is dominated by the U.S., driven by its advanced healthcare infrastructure, favorable reimbursement policies, and adoption of innovative devices. Growth is supported by large-scale manufacturers investing in R&D and direct-to-consumer platforms. Additionally, government health initiatives and early detection programs accelerate the adoption of next-generation POC devices.
With the rising incidence and prevalence of lifestyle diseases in North America, the region is gaining traction. The greater awareness of self-testing and home care kits, as well as considerable adoption of novel technologies, are expected to boost the growth of the point of care diagnostics market in the North America.
On the other hand, the Asia-Pacific is estimated to be the most opportunistic segment during the forecast period. Due to the increasing number of market players exploring the region's untapped economies, the Asia-Pacific market is expected to grow significantly over the forecast period. Furthermore, the increased use of point of care diagnostics kits in countries such as China, Japan, and India is a key driver of growth in the Asia-Pacific region.
Europe is expected to grow significantly in the point-of-care diagnostics market during the forecast period. Europe is experiencing a rise in the incidence rates of various diseases, along with the rise in adoption of new technologies. This increases the demand for the use of point-of-care diagnostics within the population. Thus, this promotes the market growth.
UK
The industries in the UK are utilizing various technological advancements in the development of new point-of-care diagnostics kits. At the same time, rising diseases are increasing their use. This increases their production, which in turn increase the collaboration among the industries.
Germany
There is a rise in the demand for the use of rapid point-of-care diagnostics approaches. Thus, new research is being conducted for their development to deal with the rising diseases. This is further supported by the government fundings.
India Point-of-Care Diagnostics Market Trends
India is emerging as one of the fastest-growing markets in the Asia-Pacific POC diagnostics sector, driven by increasing healthcare accessibility and growing awareness of chronic diseases. Government initiatives promoting preventive healthcare and rural diagnostic outreach programs further support adoption. Additionally, localized manufacturing and strategic partnerships with local distributors help accelerate market expansion.
How is the Opportunistic Rise of Latin America in the Point-of-Care Diagnostics Market?
Latin America is expected to experience notable growth in the market, driven by rising healthcare investment and increased awareness of POC diagnostics. In countries like Brazil and Mexico, regulatory reforms and stronger public-private partnerships are improving market access. Growth is further supported by expanding rural healthcare infrastructure and the increasing presence of retail health outlets. Brazil holds the largest portion of the market in Latin America, driven by Manufacturers' focus on developing affordable, region-specific products to meet local needs. Expanding urban and semi-urban healthcare infrastructure is expected to further broaden market penetration.
What Potentiates the Growth of the Point-of-Care Diagnostics market in the Middle East & Africa (MEA)?
The Middle East & Africa POC diagnostics market is expected to grow moderately, driven by improving healthcare infrastructure and rising government expenditure. Adoption is boosted by investments in telehealth and decentralized healthcare models, particularly in GCC countries. Affordability remains a concern, but the introduction of region-specific, lower-cost devices is likely to increase usage.
UAE Point-of-Care Diagnostics Market Evolution
The UAE leads the market in the Middle East & Africa, driven by strong government investment in healthcare infrastructure and advanced diagnostic services. Strategic partnerships with global manufacturers ensure the availability of technologically advanced devices. Affordable and easy-to-use tests are further gaining traction in urban and semi-urban areas.
Point-of-Care Diagnostics Market – Value Chain Analysis
- Reagents and Consumables Sourcing
The foundation of POC diagnostics lies in the supply of high-quality reagents, antibodies, enzymes, and assay kits, which are critical for accurate and rapid testing.
Key Players: Thermo Fisher Scientific, Sigma-Aldrich (Merck), bioMérieux, Sekisui Diagnostics
- Component & Device Fabrication
Raw materials and reagents are processed into components such as test strips, microfluidic chips, cartridges, and lateral flow devices used in POC instruments.
Key Players: Abbott Laboratories, Roche Diagnostics, BD (Becton, Dickinson and Company), Nova Biomedical
- Instrument / Analyzer Manufacturing
Components are integrated into portable or benchtop analyzers, handheld devices, and molecular testing platforms. Devices undergo strict calibration, quality control, and safety testing.
Key Players: Siemens Healthineers, Danaher (Cepheid, Beckman Coulter), Roche Diagnostics, Abbott Laboratories
- Software & Connectivity Integration
Advanced POC systems incorporate data analytics, connectivity to electronic health records (EHR), and cloud-based monitoring for real-time results and decision support.
Key Players: Siemens Healthineers, Roche Diagnostics, Thermo Fisher Scientific, BD
- Distribution & Deployment
Finished POC devices and test kits are supplied to hospitals, clinics, laboratories, pharmacies, and even home-care settings, supported by training and technical services.
Key Players: Abbott Laboratories, Roche Diagnostics, bioMérieux, BD, Thermo Fisher Scientific
- Post-Market Support & Maintenance
Ongoing service, software updates, calibration, and regulatory compliance support ensure device accuracy and longevity in clinical environments.
Key Players: Siemens Healthineers, Danaher, Abbott Laboratories, Roche Diagnostics
Top Companies in the Point of Care Diagnostics Market & Their Offerings
- Abbott Laboratories: Offers a massive POC portfolio featuring the ID NOW rapid molecular platform and i-STAT handheld blood analyzers for immediate bedside results.
- Bio-Rad Laboratories Inc.: Focuses on specialized POC solutions like the D-10 system for rapid diabetes monitoring and portable toxicology screening tools.
- Cardinal Health Inc.: Operates primarily as a distributor of POC diagnostic kits and supplies, providing healthcare facilities with a centralized source for various rapid testing brands.
- Cepheid:Developed the GeneXpert system, a modular platform that provides gold-standard molecular results for infectious diseases in under an hour at the point of care.
- F. Hoffmann-La Roche Ltd: Provides high-end POC systems like the cobas line for cardiac markers and infectious diseases, alongside their industry-leading Accu-Chek diabetes products.
- Mesa Biotech: Now part of Thermo Fisher Scientific, they created the Accula platform, a palm-sized PCR system used for rapid molecular detection of respiratory viruses.
- Quest Diagnostics Incorporated:Primarily a lab service provider that supports the POC market by offering management services and connectivity solutions for decentralized testing sites.
- Quidel Corporation: Now QuidelOrtho, they dominate the rapid immunoassay market with the Sofia fluorescent analyzer and QuickVue lateral flow tests.
Recent Developments
- In October 2023, EKF Diagnostics opened its new state-of-the-art life sciences manufacturing facility in the U.S. This facility is designed to address the increasing demand of an expanding customer base.(Source: https://www.ekfdiagnostics.com)
- In February 2023, bioMérieux received U.S. FDA approval for its BIOFIRE SPOTFIRE respiratory panel system to expand its POC testing product range.
(Source: https://www.biomerieux.com) - In January 2023, Cipla announced the launch of Cippoint, a point-of-care testing device with testing of cardiac markers, diabetes, infectious diseases, thyroid function, inflammation, metabolic, and coagulation markers.(Source: https://drugscontrol.org)
- In May 2025, for the detection of Respiratory Syncytial Virus (RSV), SEKISUI Diagnostics, which is a global medical diagnostics manufacturer, has launched a new rapid diagnostic testing tool in professional healthcare settings, as per the recent announcements.
- In May 2025, a Medicare reimbursement coverage for its FebriDx test, which is a rapid point-of-care (POC) diagnostic used to distinguish between bacterial and non-bacterial acute respiratory infections, from US Medicare Administrative Contractor (MAC) to Lumos Diagnostics was recently confirmed.
- In May 2025, a collaboration between Neo Medical Inc, which is an innovator in handheld ultrasound technology, and the POCUS Certification Academy, a globally recognized provider of point-of-care ultrasound (POCUS) education and Inteleos which is non-profit organization, was announced. To educate, increase proficiency, and to enhance access to advanced diagnostic technology worldwide is the aim of this collaboration.
Segments Covered in the Report
By Product
- Blood Glucose Monitoring
- Infectious Diseases
- Cardiometabolic Diseases
- Pregnancy & Infertility Testing
- Hematology Testing
- Others
By End User
- Hospital Bedside
- Physician's Office Lab
- Urgent Care & Retail Clinics
- Homecare/Self-testing
By Region
- North America
- Latin America
- Europe
- Asia-pacific
- Middle and East Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting