What is the Point-of-Care Infectious Disease Diagnostics Market Size?
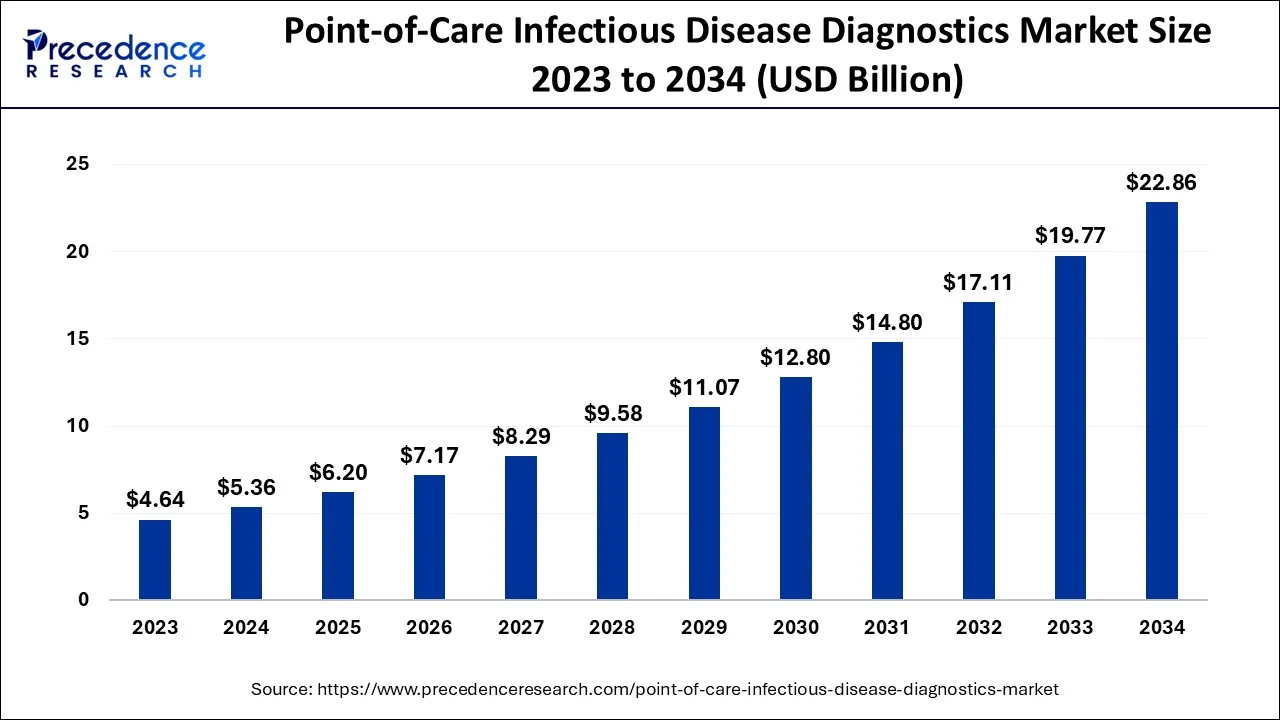
The global point-of-care infectious disease diagnostics market size is calculated at USD 6.20 billion in 2025 and is predicted to increase from USD 7.17 billion in 2026 to approximately USD 25.66 billion by 2035, expanding at a CAGR of 15.26% from 2026 to 2035.
Point-of-Care Infectious Disease Diagnostics Market Key Takeaways
- Asia Pacific region is expected to dominate the point-of-care infectious disease diagnostics market during the forecast period.
- By Technology, the lateral flow segment is expected to hold the largest share of the market throughout the forecast period.
- By Application, the hospital-associated infections (HAIs) segment is expected to hold the dominant position in the point-of-care infectious disease diagnostics market.
- By End-user, the hospital segment had the dominating share, the segment is expected to sustain the trend throughout the forecast period.
Point-of-Care Infectious Diagnostics: Transforming Healthcare with Rapid, Accessible, and Real-Time Testing Solutions
The point-of-care (POC) diagnostics market on a global level offers quick insights about patient's health at real-time and at the place of a medical interaction. As medical testing has changed due to the demand for faster test results and the development of portable, user-friendly testing equipment. As the healthcare providers focus to invest in minimal infrastructure, the market is observed to get accelerated owing to the fact that point-of-care diagnostics for infectious diseases require low cost applications that are generally easy to manage and operate.
Point-of-care diagnostics methods will remain essential in the field of medical testing as medical care services develop to become more consumer-focused in order to improve patient outcomes. Rapid outcomes from such diagnostics methods allow immediate treatment, decrease the requirement for multiple patient visits, and aid in managing infectious disease epidemics. The rising incidence of various infectious diseases across the globe will continue to highlight the demand for point-of-care diagnostics services, it was predicted by the Centers for Disease Control and Prevention that there would be between 27 and 54 million flu cases from October 2022 to April 2023.
Point-of-Care Infectious Disease Diagnostics Market Growth Factors
The demand for quick and precise diagnostic tools at the point of care has increased due to the rising incidence of infectious diseases like HIV, TB, hepatitis, and respiratory infections worldwide. Technological advancements such as molecular diagnostics, microfluidics, biosensors, and lab-on-a-chip devices have made the development of sensitive and focused point-of-care diagnostic technologies that can precisely identify infectious pathogens.
Real-time results from point-of-care diagnostics enable quick treatment initiation and lower the risk of disease transmission. Adopting such diagnostics is motivated by the demand for timely, useful information. They are instrumental in rural locations with little resources and access to centralized laboratories. These tests facilitate quick diagnosis without requiring intricate infrastructure. All these elements are observed to act as growth factors for the market.
Improving disease diagnosis and management is a significant concern for many governments and healthcare institutions. As governments and other healthcare institutions are implementing advanced diagnostic methods, the market is expected to grow at a significant rate. The trend towards home-based healthcare and telemedicine has spurred the desire for user-friendly, self-administered point-of-care tests that patients may utilize without professional assistance. Another growth factor for the market could be the rising frequency of infectious diseases among the elderly, as the world's population ages.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 | USD 6.20Billion |
| Market Size in 2026 | USD 7.71 Billion |
| Market Size by 2035 | USD 25.66 Billion |
| Growth Rate from 2026 to 2035 | CAGR of 15.26% |
| Largest Market | Asia Pacific |
| Base Year | 2025 |
| Forecast Period | 2026 To 2035 |
| Segments Covered | Technology, Application, End-user, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rise in cases of infectious diseases
The need for quick and precise diagnostic technologies that can be used at the point of care has increased due to the rising prevalence of infectious diseases such as COVID-19, HIV, hepatitis, and tuberculosis. Point-of-care diagnostics have become more popular since many conditions require early identification for efficient management and containment. There is a greater need for detection promptly to start prompt treatment and stop the spread of infectious diseases as new ones emerge and old ones reemerge. Quick data from point-of-care diagnostic equipment enables medical personnel to act quickly and strategically when managing patients.
Rapid and precise point-of-care diagnostics assist medical professionals in prescribing focused therapies, which lowers the needless usage of broad-spectrum antibiotics. In turn, this may lessen the emergence of antibiotic resistance. The drawbacks of centralized laboratory testing have been brought to light by situations such as the COVID-19 pandemic. Point-of-care diagnostics allow decentralized testing in rural or resource-limited places, contributing to more effective disease containment. Point-of-care testing gives patients more control by giving them access to quick information about their state of health. Increased patient engagement and adherence to suggested treatment plans may result from this.
Growing demand for home testing
Self-administered point-of-care diagnostic tests that can be performed at home, such as COVID-19 or HIV, are becoming increasingly common. The ease of testing at home and the desire for discretion are the driving forces behind this development of the market. The market has responded by creating user-friendly, portable testing equipment that delivers quick findings without needing a laboratory as more individuals look for simple and fast solutions to identify ailments from the comfort of their homes. This pattern has sparked advancements in point-of-care testing technologies, opening a wider choice of accessible and cost-effective solutions for diagnosing infectious diseases without the delays brought on by conventional diagnostic techniques.
Restraint
Accuracy and reliability concerns
The precision and dependability of Point-of-Care (POC) diagnostic tests might not always be on par with those of centralized lab procedures. The accuracy of results can be affected by variations in sample collection, operator abilities, and environmental factors, which may result in false positives or negatives. Diagnosing infectious diseases frequently entails complex procedures to pinpoint certain bacteria or indicators. POC tests must be easy to use, which occasionally compromises the sensitivity and specificity necessary for reliable results. It can be challenging to strike a balance between usability and clinical accuracy.
POC diagnostics are widely employed in situations with limited resources, where access to cutting-edge laboratory equipment and knowledgeable staff is constrained. The overall accuracy of diagnosis may be impacted by variances in testing methods and their interpretation. POC diagnostics frequently involve various sample types, including blood, urine, or saliva. The varying quality of multiple samples may impact the accuracy of the results. The reliability of POC tests can be affected by several variables, including sample collection, storage, and transportation.
Opportunity
Rising emphasis on rapid diagnostics for early treatment
POC diagnostics offer immediate results, enabling medical professionals to decide on a course of therapy quickly. This is crucial in situations like sepsis or bacterial infections where quick action can significantly improve patient outcomes. Rapid diagnostic tests assist medical professionals in promptly recognizing infectious diseases, enabling the timely start of effective therapies. This improves patient outcomes since conditions may be treated before they develop or cause problems. Healthcare professionals can counsel patients on preventive measures and lifestyle changes to slow the development of infectious diseases by identifying them early. This covers instruction on isolation, cleanliness, and vaccination. Early intervention can reduce the length of hospital stays, the requirement for intensive care, and overall healthcare expenses. It facilitates swift identification.
Segment Insights
Technology Insights
The lateral flow segment is expected to be dominant in the point-of-care infectious disease diagnostics market during the predicted period. Lateral flow tests are perfect for point-of-care settings when prompt judgments are essential since they produce results in only minutes. This speed makes it possible for medical professionals to launch immediate interventions and stop the spread of infectious diseases.
Lateral flow tests are portable and lightweight, making them suitable for usage in various locations, including mobile clinics, homes, and remote regions with little healthcare infrastructure. In particular, in underdeveloped areas, its portability offers greater access to diagnostics. In response to epidemics and pandemics, lateral flow testing has been crucial.
The agglutination assays segment is registered to grow faster in the point-of-care infectious disease diagnostics market during the forecast period. Agglutination assays can concurrently detect several pathogens or indicators in a single sample, resulting in a thorough diagnostic profile. This is essential for rapidly detecting co-infections or excluding particular diseases. Technology developments have produced agglutination assays that are extremely sensitive and precise. Modern tests use nanoparticles, latex beads, or magnetic particles to make agglutination responses more visible and improve diagnostic accuracy. These assays are suited for healthcare professionals with varied levels of expertise because they are simple to use and need little training. They are also flexible for use in environments with limited resources due to their simplicity.
Application Insights
The hospital-associated infections (HAIs) segment is expected to be dominant in the point-of-care infectious disease diagnostics market during the forecast period. Numerous reasons, such as rising hospitalization rates, an aging population, and the appearance of microorganisms resistant to antibiotics, have contributed to the rise in HAIs. Innovative POC diagnostic technologies that are highly accurate, simple to use and able to identify various infections have been created due to technological breakthroughs.
These techniques enable medical professionals to quickly pinpoint the HAI-causing substances. HAIs frequently need quick identification and treatment to stop them from spreading inside healthcare institutions. Point-of-care diagnostics deliver immediate results, enabling medical professionals to quickly implement infection control measures. There is an increasing need for quick diagnostic tools to help with early diagnosis and treatment of HAIs as patients' and healthcare professionals' awareness of the hazards associated with these infections grows.
The inflammatory disease segment is rapidly growing in the point-of-care infectious disease diagnostics market. The desire for quicker and easier diagnostic options has increased due to the rising prevalence of inflammatory disorders worldwide. To avoid complications, conditions including sepsis, rheumatoid arthritis, and inflammatory bowel disease demand quick detection and treatment. Point-of-care diagnostics provide real-time results, enabling prompt treatment choices and better patient outcomes. Point-of-care testing's affordability and simplicity have also aided in this market segment's expansion. Reduced turnaround times for test results lower the need for follow-up appointments and hospitalizations, which benefits patients and healthcare professionals.
End-user Insights
The hospital segment is expected to be dominant in the point-of-care infectious disease diagnostics market during the forecast period. Many individuals receive medical assistance in hospitals and primary healthcare facilities, particularly for infectious disorders. They, therefore, need quick and precise diagnostic technologies to promptly recognize and treat these disorders. It often has a well-developed infrastructure and resources, such as skilled medical staff and cutting-edge laboratory facilities, allowing it to embrace various diagnostic technologies. This decreases turnaround time for results by enabling them to conduct numerous on-site tests rather than shipping samples to outside laboratories.
The diagnostic laboratories segment shows significant growth in the point-of-care infectious disease diagnostics market during the predicted period. Technology developments have produced diagnostic tools that are more precise, quick, and user-friendly. In contrast to conventional laboratory testing, these technologies enable medical personnel to promptly detect infectious diseases at the patient's bedside and shorten the turnaround time for results. Furthermore, the COVID-19 pandemic and other infectious disease outbreaks worldwide have highlighted the critical need for quick and accessible diagnostic options. Due to this demand, money has been invested in, and research has been conducted to create cutting-edge point-of-care diagnostic technologies that can promptly identify various infectious agents, from bacteria to viruses.
The home-care settings show a notable growth in the point-of-care infectious disease diagnostics market during the forecast period. Home care settings provide patients with convenience and accessibility that is unmatched. The user-friendliness of point-of-care infectious disease diagnostic tools allows patients to conduct tests without the assistance of trained medical experts. This ease of access encourages people to take charge of their health and seek prompt testing, resulting in the early identification and treatment of infectious diseases. The demand for point-of-care infectious disease diagnostics has increased due to the increased emphasis on preventative healthcare worldwide.
Better results for individuals result from routine surveillance and early illness detection, which also helps reduce disease transmission in communities. Remote patient monitoring and telehealth have gained popularity. Healthcare providers can remotely direct patients through testing procedures using point-of-care diagnostic tools.
Regional Insights
What Makes Asia Pacific the Dominant Region in the Market?
Asia Pacific had the largest revenue share in 2023 and is expected to sustain in the point-of-care infectious disease diagnostics market throughout the predicted timeframe. Most of the world's population resides in Asia Pacific, which is also recognized to have a higher burden of infectious diseases than other areas. Contagious diseases spread because of elements including large metropolitan populations, regional healthcare disparities, and inconsistent sanitary standards. The high prevalence of diseases fuels the need for quick and convenient diagnostic tools. Asia Pacific has a wide range of geographical and climatic circumstances, which can result in infectious diseases of many kinds. This diversity calls for adaptable diagnostic approaches to address various infections and disease types. Point-of-care diagnostics provide the adaptability needed to properly handle this variability.
India Point-of-Care Infectious Disease Diagnostics Market Analysis
India's market is expanding due to high disease burden, rising demand for rapid testing in rural and remote areas, and limited access to centralized laboratories. Government screening programs, increasing public–private healthcare investments, affordability of POC tests, and growing adoption by clinics and diagnostic centers further drive market growth.
Numerous nations in the Asia-Pacific area are regarded as emerging economies, and their healthcare systems are expanding. Increasing attention is being paid to improving diagnostic skills, particularly at the point of care, as these economies grow, and their healthcare systems advance. This encourages using point-of-care infectious disease diagnostic tools that deliver quick, accurate results and support healthcare professionals in making prompt treatment choices.
The general public's growing knowledge of healthcare issues has increased the desire for quick and easy diagnostic solutions. Patients' increased proactivity in seeking medical care and diagnoses drives the demand for point-of-care infectious disease diagnostics. In Asia-Pacific, several nations have realized the value of early disease identification and management. Governments and health organizations have launched initiatives to increase healthcare access. All these elements are expected to continue the market's development throughout the forecast period.
What Potentiates the Market in North America?
North America is expected to expand in the point-of-care infectious disease diagnostics market during the forecasted timeframe. The primary driver has been the development of quick and precise diagnostic technology. At the point of care, the identification of infectious agents could be done more quickly and accurately because of advancements in molecular diagnostics, immunoassays, and biosensors. Sending samples to centralized facilities is common in traditional lab-based procedures, which delays diagnosis. Faster results from point-of-care tests enable quicker patient treatment and infection control decision-making. Self-testing and home-based diagnostics are becoming more popular. Creating simple testing kits has made it easier for people to keep track of their health, which has helped the industry grow in North America. Electronic health records (EHRs) and point-of-care diagnostics have been integrated to improve patient data management and healthcare decision-making.
U.S. Point-of-Care Infectious Disease Diagnostics Market Analysis
The U.S. market is expanding due to growing emphasis on decentralized testing, rising awareness of early disease detection, and increased adoption of portable, easy-to-use diagnostic devices. Continuous innovation in rapid test kits, integration with digital health platforms, and the need for efficient outbreak management in community and emergency care settings further fuel market growth.
What Drives the Point-of-Care Infectious Disease Diagnostics Market in Europe?
The market in Europe is expected to grow significantly due to increasing adoption of rapid testing in hospitals and clinics, rising prevalence of infectious diseases, and emphasis on early detection. Supportive government policies, investments in healthcare infrastructure, technological advancements in portable diagnostics, and the need for efficient disease outbreak management further drive market expansion across the region.
UK Point-of-Care Infectious Disease Diagnostics Market Analysis
The market in the UK is driven by the expansion of home healthcare services, rising public awareness of infectious disease prevention, and increased use of decentralized testing in remote and underserved areas. Continuous development of user-friendly, accurate POC devices and integration with digital health monitoring systems further support market expansion.
Latin America Market Trends
Latin America's market shows notable growth during the forecast period. This is due to a remarkable presence of infectious diseases like Chagas, HIV, Syphilis, Dengue, TB, and Malaria, which drives need for rapid testing. PoC tests are ideal for remote areas and even primary care, addressing shortages of skilled technicians and enhancing access.
Brazil
Brazil's market trends are driven by increasing cases of infectious diseases like Dengue, Zika, HIV, and chronic conditions, which necessitate quick, accurate testing. Patients and providers prefer faster results than conventional labs, mainly in remote areas, decreasing follow-ups and improving care. Innovations in molecular diagnostics and even multiplex assays detecting multiple pathogens improve efficiency.
MEA Market Trends
The MEA market shows a rapid growth during the forecast period. It is driven by the high prevalence of malaria, HIV, tuberculosis, and emerging infectious diseases such as COVID-19, which creates a strong demand for immediate diagnostics. Government initiatives and donor-funded programs, mainly for HIV or malaria, encourage POC adoption. Increasing investments in healthcare infrastructure and even increased access to testing are expanding the market.
UAE
UAE's market trends are driven by the rising prevalence of infectious and even chronic diseases, which fuels need for quick detection and treatment. The pandemic massively accelerated the adoption along with acceptance of PoC diagnostics for rapid screening and management. For instance, Dubai and Abu Dhabi's modern facilities, research centers, and even focus on health attract investment and need.
Value Chain Analysis of Point-of-Care Infectious Disease Diagnostics Market
- R&D
It focuses on miniaturization, improved speed, and connectivity, leveraging technologies such as biosensors, AI, microfluidics, and CRISPR to create portable, affordable apparatus for rapid, accurate, on-site results. Thus, bridging the gap between centralized labs along with patient care, mainly for outbreaks, with ongoing investment in digital integration for data management and enhanced public health response.
Key Players: Roche, Siemens Healthineers, Danaher - Clinical Trials and Regulatory Approvals
It aims on rigorous validation of analytical accuracy, clinical performance in real-world settings, and even usability by non-specialists, guaranteeing reliability for rapid, decentralized testing, with regulators such as the FDA using programs.
Key Players: Abbott, Roche Diagnostics, Siemens Healthineers, Danaher, QuidelOrtho, Thermo Fisher
Point-of-Care Infectious Disease Diagnostics Market Companies
- Danaher Corporation: Provides point-of-care diagnostic solutions, including rapid tests, immunoassays, and molecular diagnostics for infectious diseases, supporting timely patient care.
- Alcon Laboratories, Inc.: Offers ophthalmic diagnostic devices and point-of-care testing solutions, aiding in the rapid detection of ocular infections and related conditions.
- Abbott Laboratories: Delivers a wide range of POC infectious disease diagnostics, including rapid antigen, molecular, and immunoassay tests for flu, COVID-19, and other infections.
- Hoffman-La Roche Ltd: Provides rapid and molecular POC tests for infectious diseases, enabling quick detection and real-time clinical decision-making.
- Becton, Dickinson and Company: Offers rapid diagnostic platforms, biosensors, and POC testing devices for infectious diseases, enhancing patient safety and treatment speed.
- Trinity Biotech Plc: Supplies rapid POC diagnostic tests for infectious diseases, including serology and antigen detection kits for clinics and hospitals.
- Cardinal Health Inc.: Provides distribution of POC diagnostic devices, rapid test kits, and support services to healthcare facilities for infectious disease management.
Recent Developments
- In May 2024, F. Hoffman-La Roche Ltd. gained FDA approval for its HPV self-testing kit, enabling earlier detection of cervical cancer risk in women.
(Source: roche.com ) - In March 2024, SEKISUI Diagnostics received EUA approval for the OSOM Flu SARS-CoV-2 Combo Test, authorized for both professional and home testing use.
(Source: roche.com ) - In August 2023, Molbio Diagnostics and CrisprBits have established a strategic partnership as innovators in point-of-care diagnostic solutions. By integrating CRISPR into point-of-care (POC) assays, this cooperation aims to revolutionize point-of-care diagnostics.
- In May 2023, Sensible Diagnostics claimed to have created a compact, reasonably priced, sample-to-answer PCR device that could be used in 10 minutes. The company plans to introduce its system in early 2024, starting with low-cost test cartridges and initially specializing in point-of-care testing for infectious diseases.
Segments Covered in the Report
By Technology
- Lateral Flow
- Agglutination Assays
- Flow Through
- Solid Phase
By Application
- HIV
- Liver Disease
- Tropical Disease
- Inflammatory Disease
- Respiratory Disease
- Hospital Associated Infections (HAIs)
- Sexual Health Disorders
- Others
By End-user
- Hospital
- Diagnostic Laboratories
- Home-care settings
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting