Anastomosis Devices Market to Exceed USD 7.21 Bn By 2032
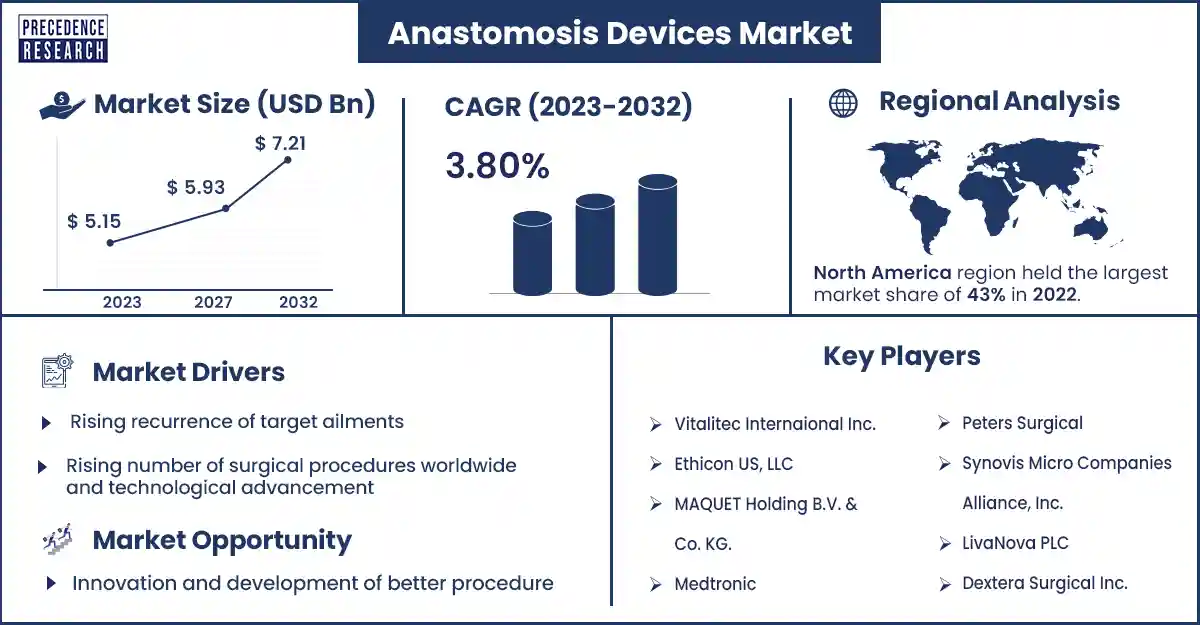
The global anastomosis devices market size was evaluated at USD 5.15 billion in 2023 and is expected to attain around USD 7.21 billion by 2032, growing at a CAGR of 3.80% from 2023 to 2032. The anastomosis devices market is attributed to the increase in lifestyle-related diseases like cancer, obesity, hypertension, diabetes, and cardiac diseases.
Market Overview
The anastomosis devices market is divided into adhesives, surgical sealants, surgical sutures, and surgical staplers. Anastomosis is a surgery in which two body channels together, such as intestines and blood vessels. Surgeons create a new anastomosis after bypassing part of a channel, and anastomosis devices are important in reducing blood loss, minimizing scarring, and facilitating quick recovery.
The increasing prevalence of gastrointestinal & cardiovascular and increasing surgical procedures fuel the growth of the market. Increasing focus on research and development capabilities in terms of adoption of modern healthcare technologies and medical devices is expected to drive the market's growth. In addition, growing expenditure for the improvement of healthcare structure and increasing the aged population to fuel the market growth. The introduction of favorable refund scenarios and technologically advanced products is also expected to drive the growth of the anastomosis devices market.
The rising number of surgical procedures worldwide and technological advancement fuel the anastomosis devices market
The rising number of surgical procedures, both emergency and elective, estimates significant growth in the market. Surgical interference requiring anastomosis behaves in several healthcare specialties, such as cardiovascular surgery, gynecology, urology, and general surgery. Major factors, including improved access, lifestyle changes, and population growth to medical services, have been responsible for the increase in surgical procedures worldwide.
In addition, the availability of skilled surgeons and technological advancements in medical infrastructure in developing markets have aided the expansion of surgical abilities, dominating a higher volume of anastomosis processes. Constant innovations in devices and surgical techniques, including the development of minimally invasive devices and robotic-assisted anastomosis procedures, are driving the market growth. The increased surgical activity enhances the requirement for anastomosis devices such as surgical sealants, sutures, and staplers. These are the major drivers responsible for the growth of the anastomosis device market.
However, limited reimbursement policies may restrain the growth of the anastomosis devices market. The lack of uniform refund policies and lack of comprehension of these medical devices in several healthcare systems are significant restraints. Due to factors including delays in reimbursement processing, strict coverage criteria, and an inadequate refund rate, reimbursement challenges are acquired. These are the few challenges that can restrain the growth of the market.
Anastomosis Devices Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 5.15 Billion |
| Projected Forecast Revenue by 2032 | USD 7.21 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 3.80% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Anastomosis Devices Market Top Companies
- Dextera Surgical Inc.
- Lydus Medical
- Johnson and Johnson
- LivaNova PLC
- Synovis Micro Companies Alliance, Inc
- Peters Surgical
- Medtronic
- MAWUET Holding B.V. & Co. KG.
- Ethicon US, LLC
- Vitalites International Inc.
Recent Development by Lydus Medical
- In January 2023, in Israel, Lydus Medical launched a microvascular anastomosis aid device for a patient's small arteries. This device was FDA clearance of vessel 510k. The aim behind this launch was to be effective, safe, and fast, enabling simple and standardized omni-vessel procedures.
Recent Development by Johnson and Johnson
- In June 2022, Johnson and Johnson launched a digitally enabled device, ECHELON 3000 Stapler, in the U.S. This innovative device allows skilled surgeons to help address their patients' creative demands. This new device is designed with 27% greater articulation time and 39% greater jaw aperture. ECHELON 3000 offers surgeons control and better access to every transaction.
Regional Insights
Asia Pacific is estimated to grow fastest in the forecast period. The rising geriatric population, growing population, growing medical care expenditures, building medical care foundations, and increasing number of clinics are major factors expected to drive the anastomosis devices market growth in Asia Pacific. India, China, South Korea, and Japan are the developing countries in the Asia Pacific region.
In China, the presence of cardiovascular and gastrointestinal surgeries are conducted due to obesity, a sedentary lifestyle, and an increasing number of people. Anastomosis devices are continuously used in these surgeries. A stapler is the major instrument in anastomosis devices, and sales of staplers in China are increasing at a higher level. The cost of staplers is much cheaper in China than in any other country.
The clinical evaluation and economic outcomes of Echelon circular power are related to manual staplers conducting left-sided colorectal anastomoses using a combination of real-world evidence and decision tree modeling in Chinese patients. These are major factors related to the anastomosis device market growth in the Asia Pacific region.
North America dominated the anastomosis devices market in 2023. The rising number of R&D research and the increasing prevalence of cardiovascular and chronic diseases are anticipated to drive market growth in North America. The United States and Canada are the emerging countries in North America's market.
In the U.S., cardiovascular and gastrointestinal diseases are the major cause of death in women, men, and aged people group and are considered for about one in every four mortalities. The major components of cardiovascular diseases cost the United States billions of dollars every year in indirect and direct values.
In these cardiovascular and gastrointestinal surgeries, staplers are used at maximum times, and they are majorly used in anastomosis devices. Hence, anastomosis devices are highly used in the United States. So, these factors are estimated to fuel the market growth of anastomosis devices in the United States.
Market Potential and Growth Opportunity
Innovation and development of better procedure
Anastomosis devices are considered to play an important role in aiming for successful and secure connections, enhancing patient outcomes, and reducing major complications. Advanced innovations such as design, materials, and technological advancements will happen in the market. Advanced innovations such as real-time imaging guidance, biocompatible materials, and robotic-assisted anastomosis will shorten operation times, minimize surgical trauma, and improve precision.
In addition, the surge in focus on minimally invasive processes will boost the demand for easily deployable, more flexible, and smaller anastomosis devices. As the industry develops, partnerships between regulatory bodies, surgeons, and medical device manufacturers will be necessary to ensure the efficacy and safety of these devices, which will help to improve patient care all over the world. These are the major opportunities that are expected to drive the growth of the anastomosis devices market in the coming years.
Anastomosis Devices Market News
- In January 2022, in Asia Pacific, for intracorporeal anastomosis, Segar Surgical Solutions Ltd. Launched the advancement of laparoscopic staplers. This company’s innovative devices offered rapid and simple closure for common enterotomy. Segar company has completed studies in human trials of devices and FsDA clearance with Waston Medical.
- In May 2023, Hiro Hasegawa and his colleagues studied a novel external reinforcement device for gastrointestinal anastomosis surgery. This innovative device is feasible and safe.
Key Market Players
- Vitalitec Internaional Inc.
- Ethicon US, LLC
- MAQUET Holding B.V. & Co. KG.
- Medtronic
- Peters Surgical
- Synovis Micro Companies Alliance, Inc.
- LivaNova PLC
- Dextera Surgical Inc.
Market Segmentation
By Type
- Reusable
- Disposable
By Application
- Gastrointestinal Surgery
- Cardiovascular Surgery
- Others
By End User
- Ambulatory Care Centres
- Clinics
- Hospitals
Buy this Research Report@ https://www.precedenceresearch.com/checkout/1858
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308