Digital PCR Market to Attain a Valuation of USD 14.28 Billion By 2032
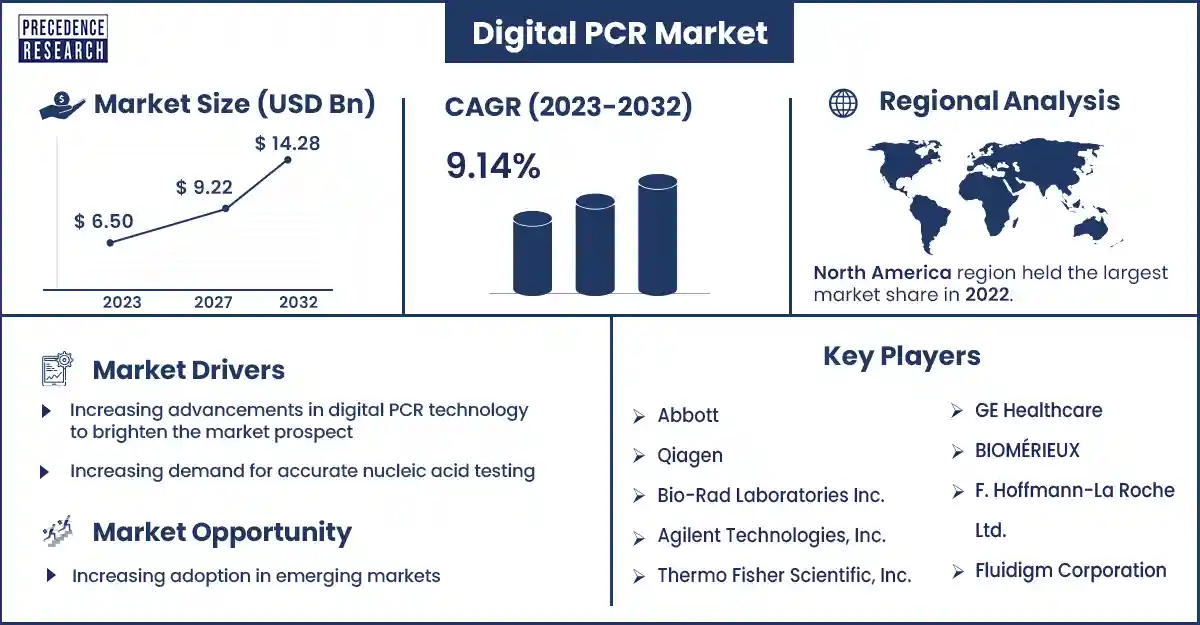
The global digital PCR market size surpassed USD 6.50 billion in 2023 and is expected to rise around USD 14.28 billion by 2032, poised to grow at a CAGR of 9.14% from 2023 to 2032. The increasing prevalence and incidence of genetic disorders and target infectious diseases are attributed to the drive of the digital PCR market.
Market Overview
The digital PCR amplifies and quantifies nucleic acids, including RNA, cDNA, or DNA, by partitioning samples into small reactions and analyzing positive ones. Digital PCR is specifically useful for applications demanding the detection of rare events, accuracy, and high sensitivity, including copy number variations or analysis of rare mutations.
The increasing recent product approvals and technological advancements, increasing use of personalized medicine, and expansion of molecular biology applications in genomics are expected to drive the market's growth. In addition, the growing integration of technology, increasing acquisitions and introduction of products by laboratories, and rising demand for liquid biopsies are further anticipated to accelerate the growth of the digital PCR market during the forecast period.
Increasing precision in nucleic acid qualification and rising demand for liquid biopsies fuels market growth
Digital PCR provides a multiple level of accuracy in the quantity of nucleic acids. Digital PCR offers more absolute quantification than traditional quantitative PCR. Accuracy is gained by separating the sample into thousands of individual reactions. Digital PCR also effectively measures the number of target molecules present. A high level of precision is necessary for several applications, including tracking viral load changes in infectious diseases, ensuring the reliability of diagnostics tests, and detecting rare genetic mutations in cancer.
In addition, liquid biopsies are included in detecting blood or different bodily fluids for the detection of cancer-associated biomarkers and are becoming significantly essential in treatment monitoring and detection of cancer at an early stage. Digital PCR’s flexibility helps in the analysis of very small amounts of mutated RNA or DNA, making it a vital technology in liquid biopsy. Professionals can use digital PCR to analyze genetic mutations related to several cancers, offering important information for customized treatment strategies. These are the major driving factors anticipated to enhance the growth of the digital PCR market.
However, some technical limitations of digital PCR may restrain market growth. A typical process of digital PCR is associated with various technical limitations, including failure to analyze target molecules due to the low quality of nucleotide template, hurdles in target detection due to the occurrence of reaction inhibitors, the need for validation and optimization of assay procedures and signal measurement during the aggressive phase of the PCR reaction. Moreover, digital PCR has various process limitations that directly impact its adoption in genomic research, such as the use of carcinogenic chemicals and the inability to assess DNA quality. These are the challenging factors that may restrain the growth of the digital PCR market.
Digital PCR Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 6.50 Billion |
| Projected Forecast Revenue by 2032 | USD 14.28 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 9.14% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Digital PCR market top companies
- Fluidigm Corporation
- GT Molecular, Inc.
- Bio-Rad
- F. Hoffmann-La Roche Ltd.
- BIOMERIEUX
- GE Healthcare
- Thermo Fisher Scientific, Inc.
- Agilant Technologies, Inc.
- Bio-Rad Laboratories Inc.
- Qiagen
- Abbott
Recent Innovation in the Digital PCR Market by Quigen
- In November 2023, Quigen launched its new software update and QIAcuity, specially designed to expand the portfolio of applications for the use of digital PCR technology in areas such as gene therapies, cell, food, and pharmaceutical safety, and RNA and DNA qualification. These advanced kits are also designed to help researchers identify two major features of digital PCR analyses.
Recent Innovation in the Digital PCR Market by GT Molecular, Inc.
- In September 2023, a company offering ultrasensitive PCR assays for cancer research, pathogen detection, and wastewater-based epidemiology, GT Molecular, Inc., launched an agreement with Roche to supply a highly multiuse respiratory panel for wastewater observation on the new Roche Digital LightCycler System. This advanced digital PCR kit evaluated respiratory pathogens such as RSV, influenza B, Influenza A, and SARS-CoV-2.
Regional Insights
Asia Pacific is expected to grow fastest during the forecast period. The increasing prevalence of infectious diseases, rising healthcare expenditure, high unmet clinical needs, and increasing investments in research and development are expected to enhance the market's growth in Asia Pacific. In addition, increasing government initiatives and demand for healthcare products to improve the population’s health also boost the growth of the digital PCR market in the Asia Pacific region.
India, China, Japan, and South Korea are the major countries in Asia Pacific that use digital PCR. China and India have the largest market share in digital PCR technology. Thermo Fisher and Bio-rad are the largest manufacturers of Chinese digital PCR. Digital microfluidic chip PCR and droplet digital PCR are the major types of digital PCR available in the Chinese market. In addition, India is a potential market for digital PCR. With the high integration of strategies focused on Indian healthcare, MNCs have addressed the affordability issue and strengthened their presence in India. These are the major factors anticipated to accelerate the market's growth in Asia Pacific.
North America dominated the digital PCR market in 2023. The growing geriatric population, increasing demand for rapid diagnostics tests, and increasing prevalence of infectious diseases, genetic disorders, and chronic diseases are attributed to North America's drive for market growth. The U.S. and Canada are the major leading countries in North America. In the U.S., various driving factors are attributed to the market's growth, such as technological advancements, including off-chip & on-chip thermocycling and microfluidic PCR in droplets.
Bio-Rad Laboratories, Thermo Fisher Scientific Inc, Hoffmann-La Roche Ltd, Fluidigm Corporation, Stilla Technologies, RainDance Technologies, Quigen N.V. and more. These are the leading market players and offer innovative digital PCR services. These are the major factors and market players anticipated to enhance the growth of the digital PCR market in North America.
Market Potential and Growth Opportunity
Increasing demand for precision medicine
Digital PCR provides sensitivity and unparalleled accuracy in quantified nucleic acids and makes it a better tool for the precise monitoring, diagnosis, and customized treatment of chronic diseases. Its capacity to detect rare genetic variations and mutations with higher precision enables it to reduce healthcare costs, enhance patient outcomes, and tailor therapeutic approaches related to trial-and-error treatments. In addition, the extending applications of digital PCR in pharmacogenomics and companion diagnostics are helping in precision medicine and further driving the growth of the digital PCR market in the coming future.
Digital PCR Market News
- In April 2024, the first ultrasensitive multiplexed digital PCR assay, the ddPLEX ESR1 Mutation Detection Kit, was launched by Bio-Rad Laboratories, Inc. The dPCR assay expands the Droplet Digital PCR, providing for the market oncology, where multiplexed mutation detection and highly sensitive assays aid disease monitoring, therapy selection, and translational research.
- In June 2023, for comprehensive quantification of up to 5 different chimeric antigen receptor CAR-T transgenes, a new multiplex digital PCR assay was launched by Navigate BioPharma Services, Inc. A Novartis subsidiary and a major supplier of advanced biomarker solutions for drug development.
Segments Covered in the Report
By Technology
- Quantitative
- Digital
- End-point
By Product
- Consumables & Reagents
- Instruments
- Software & Services
By Type
- Droplet Digital PCR
- Chip-based Digital PCR
- Others
By Indication
- Oncology
- Infectious Desease
- Genetic Disorders
- Others
By Application
- Clinical
- Research
- Forensic
Buy this Research Report@ https://www.precedenceresearch.com/checkout/2739
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308