eClinical Solutions Market Poised to Exceed CAGR 12.8% By 2032
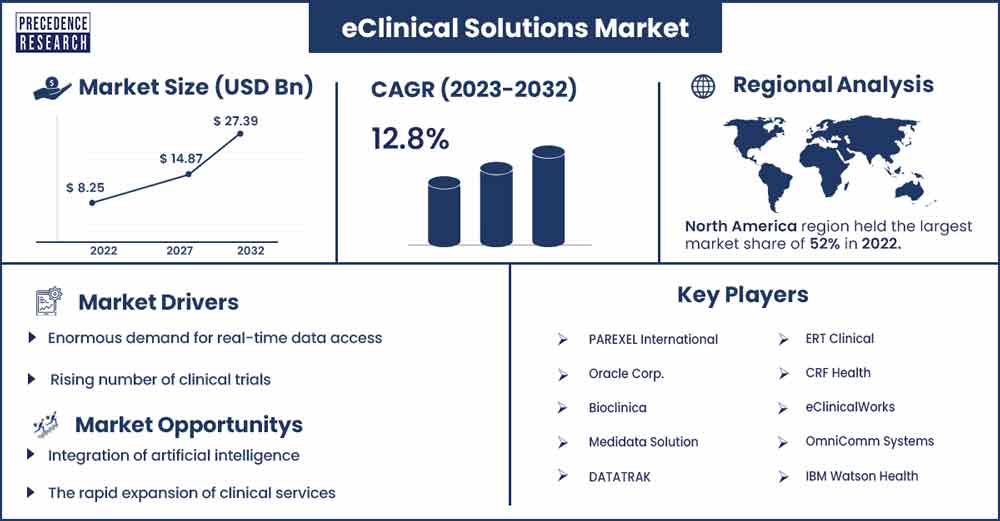
The global eClinical solutions market size was evaluated at USD 8.25 billion in 2022 and is expected to touch around USD 27.39 billion by 2032, growing at a noteworthy CAGR of 12.8% from 2023 to 2032.
Market Overview
The eClinical solutions market refers to the industry that provides technology-driven solutions and services to the clinical research and healthcare sectors. These solutions are designed to streamline and improve various aspects of clinical trials, research, and data management. Increasing healthcare infrastructure and technological advancements in the healthcare sector are driving the growth of the eClinical solution market. Clinical trial software manages the processes, operations, and data involved in the clinical studies and trials.
- There are about 452,604 registered clinical trials reported on ClinicalTrials.gov. Globally as of May 17, 2023. The report shows that there is a significant increase of the 365000 trials.
- As per the report of ClinicalTrial.gov, 77% of the registered trials are interventional with the adequate size of their percentage focus on biologics and drugs (40% of the trials or 181,721 studies) and the smaller size on devices (10% of trials or 46,809 studies).
- There were 20,109 drugs are on the research and development pipeline in 2022. The overall cost of the development of drugs in the US stands is around $2.6 billion in 2023.
Healthcare institutions like pharmaceutical companies, medical centers managed by hospitals, and medical research institutes use clinical trial solutions. Combining the software solution with clinical trials and increasing research and development programs for the clinical trials is escalating the growth of the eClinical solutions market at a significant pace.
Outsourcing of clinical trials to contract research organizations and the increasing adoption of CRO are promoting the demand for the eClinical solution. Additionally, government support and increasing demand for software solutions in clinical trials by the pharma and biopharma companies are contributing to the growth of the eClinical solution market.
Regional Insights
North America dominated the eClinical solution market in 2022. The region is expected to continue its dominance in the upcoming period. The growth of the market is attributed to the changing lifestyle preferences causing various diseases in the rising target population driving the growth of the eClinical solution market. The substantial presence of the major market players in the United States and Canada is highly contributing to the growth of the eClinical solution market. Factors like the geriatric population and rising technologies in the healthcare sector are supporting the growth of the eClinical solution market in North America.
- IBM, a major key player in the industry, released its third quarter 2023 revenue-earning results. The third-quarter revenue of $14.8 billion with an increase of 4.6% and a 3.5% increase in the constant currency.
- eClinicalWorks, a leading ambulatory cloud EHR, and healow®, EHR-agnostic, a comprehensive, cloud-based platform released their revenue. eClinicalWorks and healow® reached $800 million in 2022 and are projected to reach $900 million by 2023. And also reports the rapid growth of more than 180,000 providers.
eClinical Solutions Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 9.26 Billion |
| Projected Forecast Revenue by 2032 | USD 27.39 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 12.8% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Real-time monitoring
Real-time monitoring services in the healthcare sector assemble the data electronically and transfer it immediately from the source to the EDC system and other software systems. Remote monitoring offers several benefits such as faster identification and responses to adverse events, issues with software and devices, and missing data. Real-time monitoring data allows healthcare professionals, sponsors, and researchers to make precise decisions. E-clinical solutions are observed to offer precise real-time monitoring. The demand for real-time monitoring solutions from the healthcare industry is aimed to grow the demand for e-Clinical solutions while acting as a driver for the market.
Emergence of electronic data capture (EDC) systems
Electronic data capture (EDC) systems replace traditional paper-based methods of data collection in clinical trials, promoting more efficient and accurate data capture. This reduces the likelihood of errors associated with manual data entry, leading to improved data quality and reliability. EDC enables real-time access to clinical trial data for researchers, sponsors, and regulatory authorities. This immediate access to data allows for quicker decision-making and monitoring of trial progress. E-clinical solutions, including EDC, provide a comprehensive platform for managing and analyzing this data in real time.
Rising number of clinical trials
eClinical solution plays an important role in the implementation and management of decentralized clinical trials (DCT). The technology is useful for real-time monitoring, data collection, and communication among trial stakeholders helping to increase the data quality streamline trial processes, and improve patients' experience. Increasing use of clinical trials produces a large amount of data for analysis, eClinical solutions provide advanced analytics property for the extraction of valuable insights from the collected data. Thereby, the rise in clinical trials create a significant potential for the market to grow.
Restraints
Inaccuracy of data
E-clinical solutions are often utilized in the context of clinical trials and research studies. Inaccurate data can compromise the reliability of clinical trial results, potentially leading to incorrect conclusions about the safety and efficacy of a given treatment. This can have serious consequences for the development and approval of new drugs or medical interventions. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have stringent requirements for the accuracy and integrity of clinical trial data. Inaccuracies in data can lead to non-compliance with regulatory standards, resulting in delays in approvals or even the rejection of a drug or medical device.
Complications in adoption for rural areas
Rural and underdeveloped areas have insufficient digital infrastructure and healthcare facilities. Adoption of clinical research sites is difficult in such areas due to lack of access to the internet, computers, or smartphones which can restrict the growth of the eClinical solution market. Lack of knowledge about such services along with shortage of skilled professionals to manage the systems create a major restraint for the market.
Opportunities
Integration of artificial intelligence
AI can streamline the handling and processing of large volumes of clinical data. This is crucial for e-clinical solutions as they deal with vast amounts of information generated during clinical trials. AI algorithms can analyze historical clinical trial data to identify patterns and predict potential risks. This helps in proactive risk management and decision-making during clinical trials.
AI enables the identification of specific patient subgroups based on their genetic makeup, lifestyle, and other factors. This information is valuable for designing targeted and personalized clinical trials. The integration of AI into e-clinical solutions presents opportunities for enhancing efficiency, improving data quality, enabling personalized medicine, and ultimately accelerating the drug development process in the healthcare industry.
The rapid expansion of clinical services
Rising adoption of clinical services for better data management and development of treatment outcomes. The increasing adoption of clinical trials tends to the higher collaboration with big pharmaceutical organizations, CROs, research institutes, technology providers, and regulatory bodies. With the rising collaboration and alliances, there are adequate opportunities for the growth of the eClinical solution market in the upcoming period.
Recent Developments
- In October 2023, a major provider of life science solutions announced the collaboration with the full-service CRO “Atherion Bioresearch”. The strategic partnership aims to enhance the opportunities for organizations to increase and streamline processes, grow, and improve data integrity.
- In October 2023, Almac Clinical Technologies announced the partnership with Exostar aiming to assist the risk associated with the intellectual property. According to the collaboration, the organizations will come to provide Federated Authentication Access and Single Sign-On (SSO) to eClinical applications for supporting clinical trials which also includes Interactive Response Technology (IRT).
- In October 2023, a healthcare technology organization “Madnet” launched the latest software release having (eConsent) electronic consent capabilities in the iMednet eClinical platform. The launch was designed to generate a cost-effective, simple, and general method to collect and store participants' consent for fulfilling the virtual, hybrid, and decentralized clinical trials.
Major Key Players:
- PAREXEL International
- Oracle Corp.
- Bioclinica
- Medidata Solution
- DATATRAK
- ERT Clinical
- CRF Health
- eClinicalWorks
- OmniComm Systems
- IBM Watson Health
- eClinical Solutions
Market Segmentation:
By Product
- EDC & CDMS
- eCOA
- Clinical Data Integration Platforms
- CTMS
- Safety Solutions
- RTSM
- Clinical Analytics platforms
- eTMF
By Development Phase
- Phase IV
- Phase III
- Phase II
- Phase I
By Delivery Mode
- Licensed Enterprise
- Web-hosted
- Cloud-based
By End-Use
- CROs
- Hospitals
- Academic Institutes
- Medical Device Manufacturers
- Pharma & Biotech Organizations
Buy this Research Report@ https://www.precedenceresearch.com/checkout/1092
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 9197 992 333