Oncolytic Virus Therapy Market Revenue to Attain USD 9.85 Bn by 2033
Oncolytic Virus Therapy Market Revenue and Trends 2025 to 2033
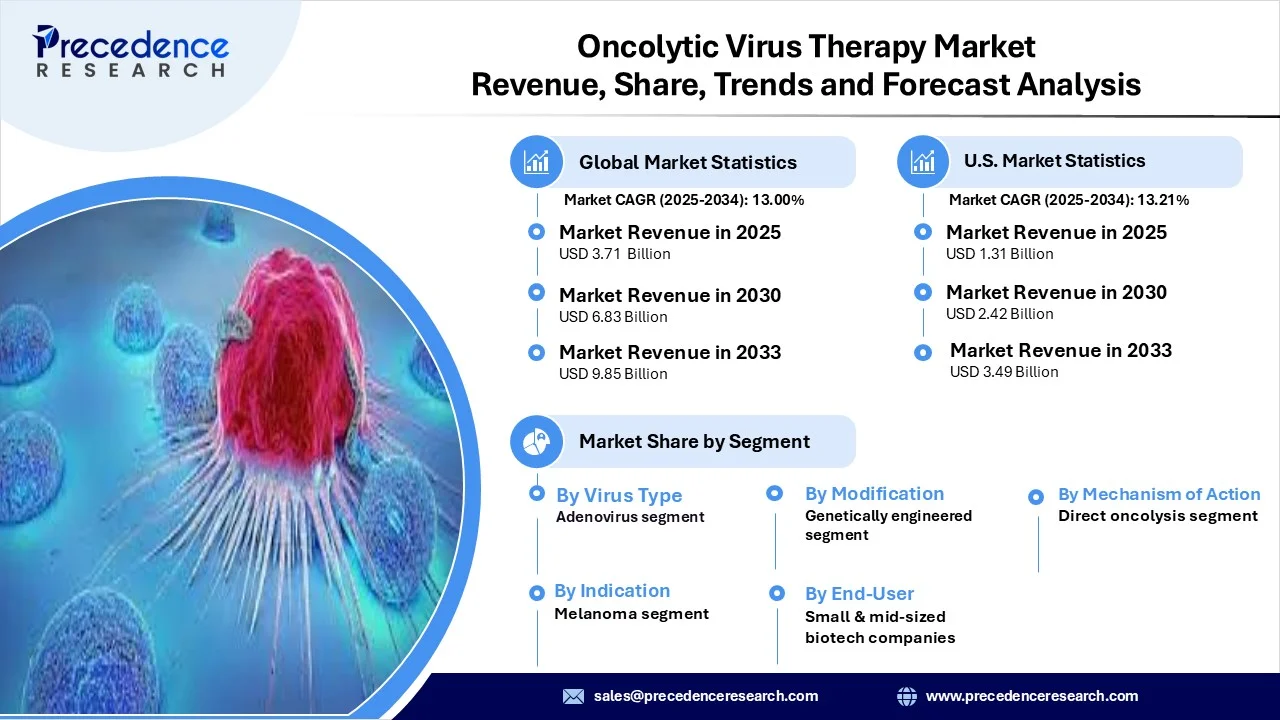
The global oncolytic virus therapy market revenue reached USD 3.71 billion in 2025 and is predicted to attain around USD 9.85 billion by 2033 with a CAGR of 13.00%. This market is expanding because it offers a promising dual-action approach, selectively infecting and lysing tumor cells while simultaneously triggering anti-tumor immune responses, addressing limitations of conventional cancer therapies.
What are the key factors driving the growth in this market?
The increasing prevalence of cancer, especially treatment-resistant and metastatic forms, is driving demand for innovative cancer therapies like oncolytic viruses (OVs). This, along with rising investments in biotechnology and gene therapy, has boosted funding for R&D, clinical trials, and commercialization. Advances in viral vector engineering, synthetic biology, and delivery methods have improved the specificity, safety, and effectiveness of OVs. Furthermore, strategic partnerships, licensing agreements, and established regulatory pathways are expediting development timelines. The growing acceptance of immuno-oncology and the combination of OVs with checkpoint inhibitors or CAR-T therapies offer significant synergies, expanding potential applications and market opportunities.
Segment Insights
- By virus type, the adenovirus segment dominated the market in 2024, owing to its well-characterized biology, ease of genetic modification, favorable safety profile, and tumor-targeting capabilities.
- By modification / engineering, the genetically engineered segment dominated the market in 2024, as these viruses can be tailored with immunomodulatory genes, tumor-specific promoters, and safety switches, enhancing therapeutic efficacy and minimizing off-target effects.
- By mechanism of action, the combined direct oncolysis segment dominated the market, as dual mechanisms promote stronger tumor control than either direct lysis or immune stimulation alone.
- By indication / type of cancer, the melanoma segment dominated the oncolytic virus therapy market in 2024, due to the fact that the first approved oncolytic virus was targeted for melanoma, and there are many trials focused on skin cancers with accessible lesions.
- By end user, the small & mid-sized biotech companies/startups segment led the market, as they are typically at the forefront of innovation and early-stage development before partnering with larger pharma firms.
Regional Insights:
North America registered dominance in the oncolytic virus therapy market, holding the largest share in 2024. This is mainly due to its well-established biotechnology ecosystem, significant R&D funding in oncology, and a robust regulatory framework. The U.S. is at the forefront of oncolytic virus therapy adoption, thanks to its existing infrastructure, reimbursement systems, and the presence of major industry players.
Asia Pacific is expected to experience the fastest growth, fueled by a rising cancer burden, expanding biomanufacturing capabilities in China, Japan, and South Korea, and increasingly favorable regulatory frameworks. Early approvals and growing local funding are also contributing to the momentum of adoption.
Oncolytic Virus Therapy Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 3.71 Billion |
| Market Revenue by 2033 | USD 9.85 Billion |
| CAGR from 2025 to 2033 | 13.00% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa |
Oncolytic Virus Therapy Market key Players
- Amgen Inc. (with Imlygic / T VEC)
- Replimune Group Inc.
- Oncolytics Biotech Inc.
- Sorrento Therapeutics, Inc.
- PsiOxus Therapeutics Ltd.
- Transgene SA
- SillaJen, Inc.
- Lokon Pharma AB
- CG Oncology Inc.
- DNAtrix
- Targovax
Recent Development
- In August 2025, Calidi Biotherapeutics’ CLD-201 (a stem cell-loaded oncolytic virus candidate for soft tissue sarcoma) was granted Fast Track designation by the FDA, accelerating its clinical development. (Source- https://www.labiotech.eu)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6939
You can place an order or ask any questions, please feel free to contact us at sales@precedenceresearch.com |+1 804 441 9344