PEEK Interbody Devices Market Revenue to Attain USD 4.72 Bn by 2033
PEEK Interbody Devices Market Revenue and Trends 2025 to 2033
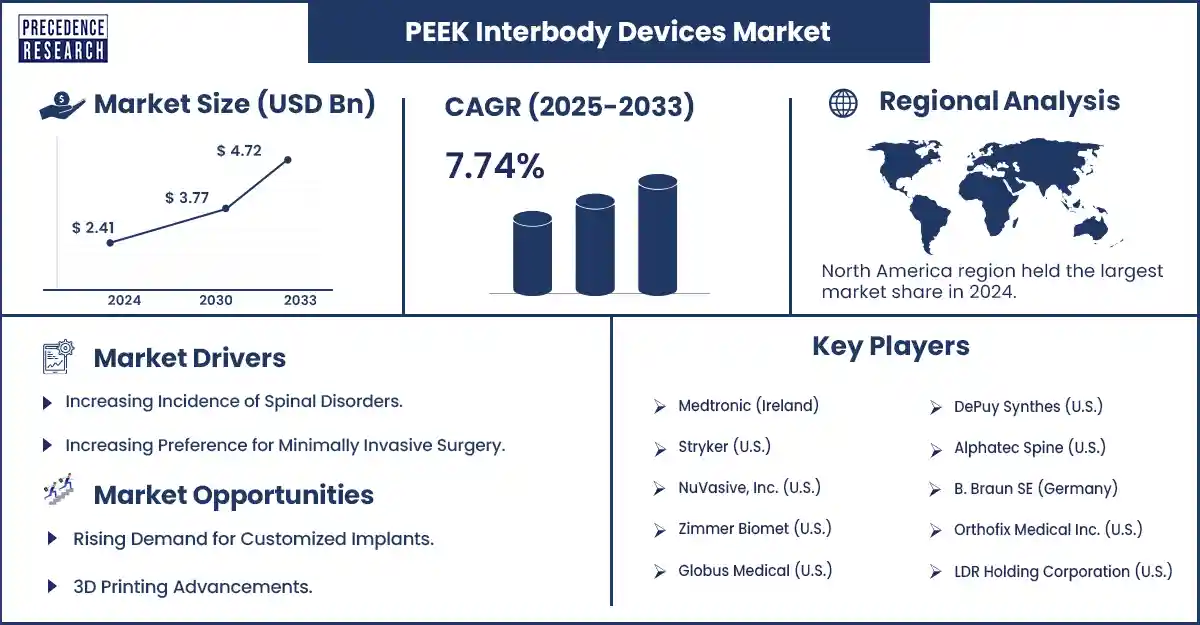
The global PEEK interbody devices market revenue reached USD 2.6 billion in 2025 and is predicted to attain around USD 4.72 billion by 2033 with a CAGR of 7.74%. The growth of the market is attributed to the increasing prevalence of spinal disorders and demand for biocompatible, minimally invasive options that promote healing and ensure long-term stability.
Exploring the Growth Potential of the PEEK Interbody Devices Market
The market is experiencing robust growth, driven by many factors. One of the primary drivers is the increasing prevalence of spinal disorders, primarily degenerative disc disease and spinal stenosis. The growing aging population across the globe is contributing to market growth. Also, the increasing patient preference for minimally invasive spinal surgeries continues to increase the demand for PEEK interbody devices, as PEEK offers great biocompatibility, radiolucency, and mechanical properties similar to natural bone.
As PEEK interbody devices allow for bone fusion and post-op images without compromising the surgical and post-op planning process, surgeons are increasingly adopting PEEK interbody devices. There are many advancements in spinal implant technology (e.g. 3D printing, surface-modifying technologies, etc.) that allow for enhanced performance and quicker acceptance of PEEK spinal implants as viable alternatives to traditional prosthetics and other technological solutions. Additionally, rising medical expenditures, increasing awareness regarding spinal health, and favorable reimbursement policies in developed regions positively impact the market. The rising ambulatory spine procedures and a preference for faster recovery solutions reinforce PEEK interbody devices as a preferred solution in modern spine care, positively impacting global market growth.
Segment Outlook
- By product type, the interbody fusion devices segment held the biggest share of the market in 2024. The segment’s dominance is mainly attributed to the increased incidence of spinal disorders and spinal fusion surgeries. Minimally invasive procedures are increasingly being performed using interbody fusion devices because of their strength, stability, and biocompatibility. Advancements in surgical technology and medical devices lead to better surgical outcomes using interbody fusion devices
- By end-user, the hospitals segment led the market, holding the largest share in 2024. This is mainly due to the higher patient inflow for spinal procedures and increased adoption of new and innovative PEEK-based implants from orthopedic and neurosurgeons.
Regional Insights
North America held the largest share of the PEEK interbody devices market in 2024. The U.S. and Canada emerged as a major player in the region due to their advanced healthcare system and high adoption of advanced surgical technologies. The increased number of patients who underwent spinal surgery further bolstered regional market growth.
Asia Pacific is expected to experience the fastest growth in the coming years. Improvements in healthcare infrastructure, particularly in China, India, Japan, and South Korea, increase in medical tourism, and rising awareness of spine care are boosting the growth of the market. In addition, the rising number of cases of spinal cord disorders is likely to boost regional market growth.
PEEK Interbody Devices Market Coverage
| Report Attribute | Key Statistics |
| Market Revenue in 2025 | USD 2.6 Billion |
| Market Revenue by 2033 | USD 4.72 Billion |
| CAGR from 2025 to 2033 | 7.74% |
| Quantitative Units | Revenue in USD million/billion, Volume in units |
| Largest Market | North America |
| Base Year | 2024 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Recent Development
- In September 2024, Nvision Biomedical and Invibio earned FDA clearance for the first 3D‑printed porous PEEK‑OPTIMA cervical and ALIF interbody system, designed with additive porous structures to enhance bone ingrowth.
(Sources- https://www.victrex.com)
PEEK Interbody Devices Market Companies
- Medtronic (Ireland)
- Stryker (U.S.)
- NuVasive, Inc. (U.S.)
- Zimmer Biomet (U.S.)
- Globus Medical (U.S.)
- DePuy Synthes (U.S.)
- Alphatec Spine (U.S.)
- B. Braun SE (Germany)
- Orthofix Medical Inc. (U.S.)
- LDR Holding Corporation (U.S.)
- SpineArt (Switzerland)
- Mazor Robotics (Israel)
- Invibio (UK)
- Raymedica (U.S.)
- K2M (U.S.)
- Finceramica (Italy)
- Biomet (U.S.)
- Exactech (U.S.)
- Japan Medical Materials (Japan)
- Curiteva (U.S.)
Get this report to explore global market size, share, CAGR, and trends, featuring detailed segmental analysis and an insightful competitive landscape overview @ https://www.precedenceresearch.com/sample/6254
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com|+1 804 441 9344