Pharmaceutical Sterile Sample Bags Market Set for Strong Growth by 2034
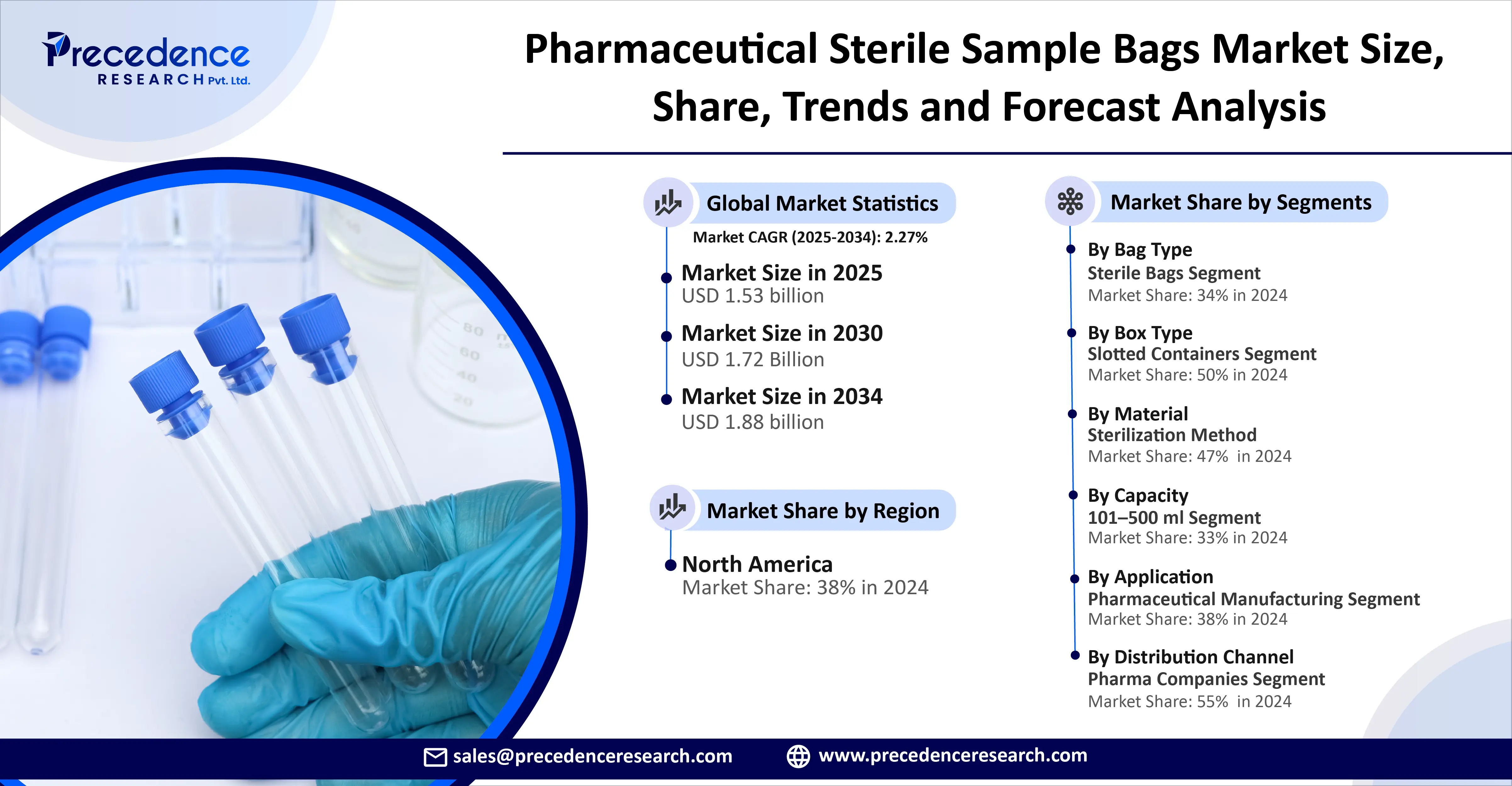
According to Towards Packaging, the global pharmaceutical sterile sample bags market size was estimated at USD 1.50 billion in 2024 and is anticipated to reach around USD 1.88 billion by 2034, growing at a CAGR of 2.27 from 2025 to 2034. The emerging need for sterile sample storage solutions in the pharmaceutical industries is due to strict regulatory compliance and emphasis on quality control.
Global Outlook
The pharmaceutical sterile sample bags market is an integral part of the pharmaceutical industry due to compliance with food safety standards and regulations. The sterile sample storage bags are essential to prevent contamination during sampling and maintain sample integrity for accurate testing. These bags are versatile in their industrial applications: sampling raw ingredients, testing finished food products, and surface sampling for hygiene monitoring. They also exhibit broad applications beyond pharmaceutical industries, including their uses in medical testing, research and environmental sampling, storage, and transport of liquids and semi-solids. The top companies that offer various sterile sample bags include 3M Company, Sartorius AG, Thermo Fisher Scientific Inc., Labplas Inc., and many others.
Market Opportunity: Emphasis Over Quality Control
The various regulatory agencies like the U.S. Food and Drug Administration, the European Medicines Agency, and the World Health Organization are introducing stringent regulatory standards. They are resulting in high emphasis on quality control and regulations. The pharmaceutical companies are adopting advanced sterile sampling solutions and systems. The companies are focusing on delivering eco-friendly and environmentally sustainable solutions.
- In May 2024, Maiva Pharma raised Rs. 1,000 Crores from Morgan Stanley PE and India Life Sciences Fund to establish a new manufacturing facility near Karnataka’s Hosur with capabilities in oncology, bags, pre-filled syringes, sterile dosage forms, and hormonal injectables.
Key Growth Factors
- The increased pharmaceutical production and growing emphasis on hygiene and safety drive technological advancements in biopharmaceutical companies.
- The convenience and ease of use of single-use bags make them popular among global end users.
- The increased investments in R&D and growth in end-use industries like food processing, biotechnology, and pharmaceuticals foster the growth of the pharmaceutical sterile sample bags market.
Market Restraint: Stringent Regulatory Stndards
It is challenging for biopharmaceutical companies to meet stringent regulatory standards, maintain sterility, and prevent contamination. The companies face potential concerns related to cost and material innovation due to high initial investment costs and fluctuations in raw material prices. There is intense competition between established and emerging market players to drive innovations by creating a high burden on pricing.
Segmental Outlook
Bag Type Insights
The stand-up sterile bags segment dominated the pharmaceutical sterile sample bags market in 2024 owing to several advantages of stand-up sterile bags in laboratory and sampling settings. These bags offer enhanced efficiency, hands-free operation, cost-effectiveness, durability, and safety. The stand-up sterile bags are ideal to ensure sterility, identify clear samples, and achieve sustainability.
Material Insights
The polyethylene (PE) segment dominated the pharmaceutical sterile sample bags market in 2024 due to the exceptional purity and sterility of pharma-grade polyethylene and its manufacturing under stringent quality control measures. Polyethylene offers excellent barrier properties against water vapor and moisture which protect sensitive samples from degradation. Medical-grade polyethylene is biocompatible and safe, which reduces the risk of harmful reactions.
Sterilization Method Insights
The gamma irradiation segment dominated the pharmaceutical sterile sample bags market in 2024 owing to the compatibility and non-thermal nature of gamma irradiation for sterile sampling bags. It offers efficiency, scalability, sterility assurance, and effective sterilization. It effectively eliminates bacteria, viruses, and other microorganisms through their DNA damage and reproduction prevention.
Capacity/ Volume Insights
The 101–500 ml segment dominated the pharmaceutical sterile sample bags market in 2024 due to the optimal sample volume and maintenance of sterility. This capacity/ volume ensures enhanced sample integrity and reduced cross-contamination risk. These bags are easy to handle and lightweight in nature and allow efficient sampling.
End-Use Application Insights
The pharmaceutical manufacturing segment dominated the pharmaceutical sterile sample bags market in 2024 owing to the increased focus on aseptic sampling and sample protection during transport. The manufacturing process ensures accurate sample testing and compliance with regulations. The industries maintain sample integrity and sterility through good manufacturing practices.
Geographical Outlook
North America
North America dominated the pharmaceutical sterile sample bags market owing to robust pharmaceutical manufacturing and R&D. The Pharmaceutical Research and Manufacturers of America introduced a new policy agenda to achieve a healthier America. In February 2024, Pfizer made a partnership with the American Cancer Society to fill the gaps in cancer care disparities through the ‘Change the Odds’ initiative. It is a 3-year initiative driven by $15 million funding support. Trump has recently signed an order to reduce U.S. drug prices in May 2025. The U.S. FDA launched the advanced manufacturing technologies designation program and also established the Innovative Science and Technology Approaches for New Drugs.
- In April 2025, Novartis planned to invest $23 billion in the expansion of its US-based R&D and manufacturing facility over the next 5 years.
The U.S.
The U.S. Food and Drug Administration conducts drug quality sampling and testing programs due to the highest standards for approval of new and generic drugs and biologics across the globe. In August 2025, the U.S. FDA announced a new FDA PreCheck program to accelerate drug manufacturing in the U.S. The U.S. Generic & Biosimilar Medicines is aiming to protect patients for the next 40 years and beyond by considering the importance of the generic and biosimilar industry.
Asia Pacific
Asia Pacific is expected to emerge at the fastest CAGR in the pharmaceutical sterile sample bags market during the forecast period of 2025 to 2034, owing to the rising healthcare expenditure and increasing pharmaceutical manufacturing activities. This region has a well-established economy, diverse healthcare systems, and cultural differences. The various nations work together to meet the manufacturing needs of medical products, which has created new opportunities and challenges for the pharmaceutical industry. China’s government has also implemented significant measures. China’s drug regulatory administration system allows pharmaceutical development that can better meet patient needs in China.
Strategic Moves by Key Players
- In October 2024, Merck Millipore invested $76 million in the expansion of antibody drug conjugate manufacturing capabilities for new cancer therapies.
- In March 2025, Sartorius AG planned to invest in a new production line in India for pallet tanks used in single-use bioreactors at its R&D facility in Bengaluru by 2027 to meet global and local demands.
Pharmaceutical Sterile Sample Bags Market Companies
- Merck Millipore
- Saint-Gobain
- Single Use Support
- West Pharmaceutical Services
- Bürkle GmbH
- Uniflex Healthcare
- Inteplast Group
- Avantor (VWR)
- Labplas Inc.
- 3M Company
- Sartorius AG
- Thermo Fisher Scientific Inc.
- Whirl-Pak
Pharmaceutical Sterile Sample Bags Market Segments covered in the report
By Bag Type
- Stand-Up Sterile Bags
- Flat Sterile Bags
- Ziplock / Reclosable Sterile Bags
- Tamper-Evident Sterile Bags
- Valve & Ported Sterile Bags
- Custom/Compartmentalized Bags
By Material
- Polyethylene (PE)
- Polypropylene (PP)
- Nylon
- Multi-layer Films (PE/PA, PE/PP composites)
- Medical-Grade Laminates
By Sterilization Method
- Gamma Irradiation
- Ethylene Oxide (EtO) Sterilization
- Steam/Autoclave Sterilization
- Electron Beam (E-Beam) Sterilization
By Capacity / Volume
- Up to 100 ml
- 101–500 ml
- 501 ml – 1 liter
- 1 – 5 liters
- Above 5 liters
By End-Use Application
- Pharmaceutical Manufacturing
- Clinical Trials & Research Labs
- Biotechnology & Biologics Facilities
- Hospitals & Diagnostic Centers
- Contract Development and Manufacturing Organizations (CDMOs)
- Regulatory & Quality Assurance Testing Labs
By Region
- North America
- U.S.
- Canada
- Mexico
- Asia Pacific
- China
- Singapore
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Source: https://www.towardspackaging.com/insights/pharmaceutical-sterile-sample-bags-market-sizing