Systemic Inflammatory Response Syndrome Treatment Market Size, Growth Report 2027
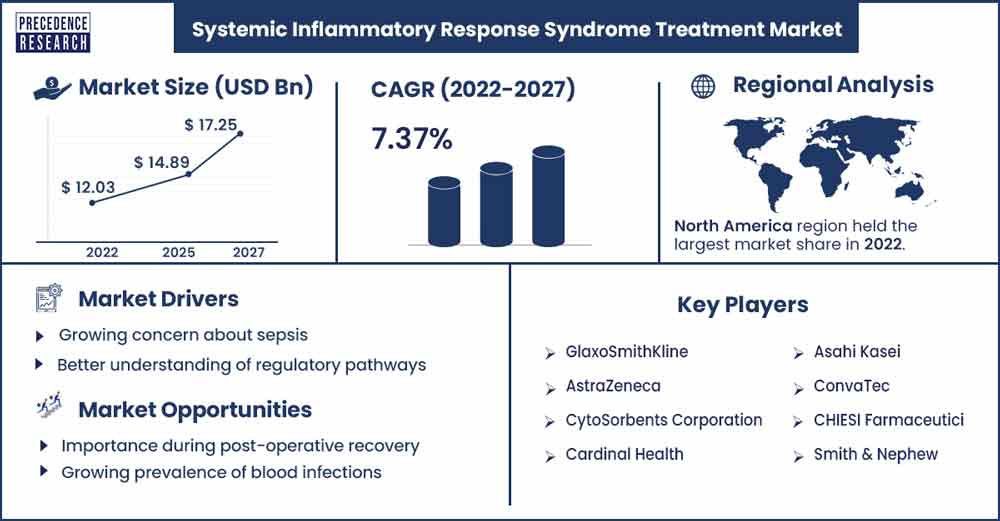
The global systemic inflammatory response syndrome treatment market size surpassed USD 12.03 billion in 2022 and is expected to rake around USD 17.25 billion by 2027, poised to grow at a CAGR of 7.37% from 2022 to 2027.
Market Overview
Systemic inflammatory response syndrome (SIRS) is the body's heightened defensive reaction to harmful stressors such as malignancy, infection, trauma, surgery, acute inflammation, and ischemia. The primary goal is to identify and eliminate the source of the insult, whether it originates from within the body (endogenous) or comes from an external source (exogenous). It's crucial to provide proper care for individuals afflicted with SIRS. This involves ensuring they can breathe well and have enough oxygen, giving fluids to improve hydration, and maintaining blood pressure. If there's a bacterial infection, antibiotics may be used. In some cases, medications that suppress the immune system, like steroids, might be necessary. Severe cases may require hospitalization in the intensive care unit for things like mechanical ventilation and close monitoring. Treating SIRS involves teamwork with doctors, nurses, and other healthcare professionals working together. The main goals are to reduce organ problems, address the root cause, and improve overall patient outcomes.
More infections and health issues are happening more often, leading to a higher need for treatments. Antibiotic-resistant infections are prevalent in people with weakened immune systems, and an increase in severe burns and trauma cases contributes to more cases of SIRS. Due to this, there's a growing demand for effective treatments to control the body's inflammatory reaction and stop the disease from spreading. This has led to a focus on developing new ways to diagnose and treat SIRS, and it's expected to expand the market.
The market for drugs treating systemic inflammatory response syndrome is growing because healthcare professionals have a significant demand to identify and treat SIRS early. Early diagnosis and treatment are crucial to preventing the disease from worsening and improving patient outcomes. So, there's a focus on training medical staff to recognize the signs of SIRS and start the proper treatment quickly. Because catching it in advance is a crucial step of the treatment process, there's a greater need for diagnostic tools and treatment options. As more people become aware of SIRS, the demand for related healthcare services and products is increasing, driving the market's growth. The creation of many upcoming drugs results from substantial efforts backed by government and private funding.
The rise of personalized medicine highlights the importance of customising treatments for each individual. This has resulted in the creation of targeted therapies and diagnostic tools based on biomarkers, enhancing the effectiveness of personalized treatment for the condition. Additionally, there is a growing trend of collaboration among pharmaceutical companies, research institutions, and healthcare providers. These partnerships aim to jointly create innovative treatment options for SIRS, speed up the discovery and development of new therapies, and ultimately benefit patients globally.
Advancements in technology have resulted in the development of new and unique diagnostic tools, aiding medical professionals in quicker and more accurate diagnosis of Systemic Inflammatory Response Syndrome (SIRS). The growing demand for these devices is expected to contribute to the market's continued expansion. For instance, The introduction of innovative therapeutic options, such as immunosuppressive drugs and targeted therapies, has increased the need for effective SIRS treatments that can manage the inflammatory response and slow down the progression of the disease. Consequently, there is a greater emphasis on research and development, which is anticipated to support market growth in the coming years.
- A study from the University of Colorado School of Medicine found that out of 56,210 ER visitors in a year, 40,356 were kids who fit the study criteria. Nearly 93% of visits with high fever (above 38.5°C) showed signs of SIRS among these.
- In a study by the American Academy of Neurology, they found that out of 780 patients with intracerebral hemorrhage (ICH), about 22% experienced non-infectious SIRS, and around 37% developed sepsis while in the hospital.
- Adrecizmab, developed by Adrenomed AG. It's a valuable treatment for septic shock, effectively treating it, preventing vascular damage, and maintaining proper vascular functions.
Regional Snapshot
North America dominates the market for treating systemic inflammatory response syndrome due to the increasing prevalence of SIRS in the region. The growing awareness of SIRS among healthcare professionals worldwide contributes to industry growth. Moreover, the presence of key industry players in North America and strategic initiatives implemented by major regional companies are expected to drive significant growth in the SIRS treatment market shortly.
- In March 2023, CytoSorbents Corporation (CTSO), known for its expertise in treating severe medical conditions in intensive care and cardiac surgery through blood purification, revealed its scientific agenda for the 42nd International Symposium on Intensive Care & Emergency Medicine.
- In November 2023, Cardinal Health's Monoject syringes received an unfavourable recommendation from the FDA. This decision followed reports of treatment delays and inaccurate therapy (either overdose or underdose) when the syringes were used with a syringe pump or a patient-controlled analgesia (PCA) pump.
Systemic Inflammatory Response Syndrome Treatment Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 12.9 Billion |
| Projected Forecast Revenue by 2027 | USD 17.25 Billion |
| Growth Rate from 2022 to 2027 | CAGR of 7.37% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2022 to 2027 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Growing concern about sepsis
As concerns grow about the prevalence of sepsis in systemic inflammatory response syndrome, industry players are actively working on developing innovative and effective drugs and therapeutics. CytoSorb, a specific extracorporeal cytokine adsorber, is emerging as a successful treatment for reducing inflammation and managing the failure of vital organs like the lungs, kidneys, heart, and brain, among other therapies. Additionally, biomarkers for early detection, treatment, and resolution of the condition have been introduced, responding to the increasing demand for early-stage diagnosis of sepsis.
Better understanding of regulatory pathways
Recognizing that various injuries trigger a typical inflammatory response in the body, researchers have identified promising targets for new anti-inflammatory treatments. These treatments aim to provide specific care or prevent further inflammation. To effectively apply these strategies, early clinical markers are crucial for real-time evaluation, helping to identify patients likely to respond well to targeted interventions. Researchers emphasize the importance of interrupting inflammatory pathways early for successful outcomes. If septic patients can be identified in the initial phase, this approach holds the potential for achieving the desired benefits and can boost the market growth.
Restraint
Regulatory and financial limitations
The market for treating systemic inflammatory response syndrome faces several challenges, mainly due to the high cost of treatment, making it unaffordable for a significant portion of the population. Limited awareness of SIRS and its symptoms can lead to delayed diagnosis and treatment, worsening the condition. The complexity of the illness and its treatment poses obstacles to developing effective medications. Stringent regulatory requirements and the high costs associated with clinical studies may restrict the development and approval of new medicines, thereby restricting the market for SIRS treatments.
Opportunities
Importance during post-operative recovery
Systemic inflammatory response syndrome is crucial in evaluating complications and organ dysfunction after surgery. The progression of this syndrome is associated with extended hospital stays, a higher risk of multiple organ dysfunctions, and increased morbidity. In cardiac surgery, SIRS is a common post-operative complication that can lead to organ failure or even death. There's an anticipated rise in the prevalence of SIRS among pediatric patients shortly. Major industry players are continuously developing effective therapies and drugs for treating SIR. Industry significant growth opportunities in this field.
Growing prevalence of blood infections
The revenue generated per patient for treating sepsis is notably high, with the average cost per hospital stay being twice that of other diseases. Sepsis is a significant cause of hospital readmission, contributing to a cost exceeding $3.5 billion in the United States. According to a recent study in the CHEST Journal, the estimated annual cost of sepsis readmissions is nearly 50% of the combined costs of the four conditions covered by Medicare's Hospital Readmissions Reduction Program (HRRP), which include acute myocardial infarction, pneumonia, chronic obstructive pulmonary disease, and congestive heart failure. The increasing prevalence of bloodstream infections is expected to drive a greater adoption of sepsis diagnostic products.
Recent Developments
- In October 2023, the medical research organization and charity LifeArc unveiled a new initiative. This program aims to invest over £100 million by 2030 to enhance the quality of life for individuals affected by rare diseases.
- In July 2023, Bristol Myers Squibb received European Commission approval for Opdivo (nivolumab) in combination with platinum-based chemotherapy for the neoadjuvant treatment of resectable non-small cell lung cancer in adult patients at high risk of recurrence with tumor cell PD-L1 expression of 1% or higher.
Kay Market Players
- GlaxoSmithKline
- AstraZeneca
- CytoSorbents Corporation
- Cardinal Health
- Asahi Kasei
- ConvaTec
- CHIESI Farmaceutici
- Smith & Nephew
Market Segmentation
By Product Type
- Urinary Tract Infection (UTI)
- Autoimmune Diseases
- Meningitis
- Pneumonia
- Others
By Application
- Specialty Clinics
- Hospital & Ambulatory Surgical Centers
- Others
Buy this Research Report@ https://www.precedenceresearch.com/checkout/1245
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308