Transcatheter Aortic Valve Replacement Market Size, Growth, Report 2032
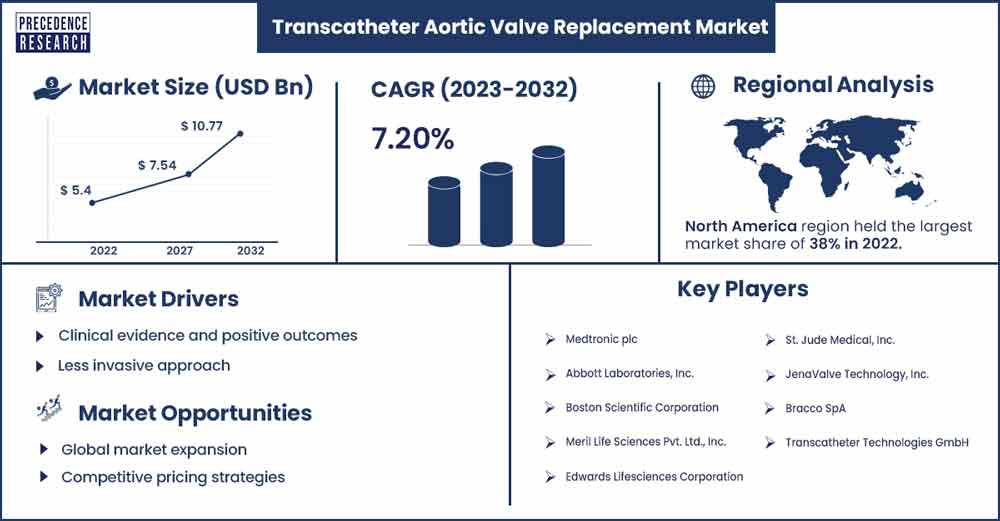
The global transcatheter aortic valve replacement market size surpassed USD 5.4 billion in 2022 and is anticipated to reach around USD 10.77 billion by 2032, growing at a CAGR of 7.2% from 2023 to 2032.
Market Overview
The market for transcatheter aortic valve replacement (TAVR) refers to the commercial environment around creating, producing, and distributing TAVR-related products and technology. Usually, they consist of artificial heart valves that may be inserted into the aortic valve location using catheters and are frequently guided by imaging methods like fluoroscopy. The demand for TAVR surgeries has increased as the surgery becomes more widely acknowledged and patients look for less intrusive solutions.
Innovations could include improvements in the materials utilized in the devices, imaging methods, distribution systems, and valve design. Market dynamics are impacted by the regulatory and approval procedures for TAVR devices. Regulations, health authority approvals, and reimbursement policies can all have a significant impact on the market.
Age-related increases in the prevalence of aortic valve stenosis are anticipated, as is the need for less invasive procedures like TAVR as the world's population ages. Advancements improve the safety and effectiveness of TAVR operations in imaging technology, delivery systems, and device design. Patients and healthcare professionals are more accepting of TAVR because of encouraging clinical results and data demonstrating its safety and effectiveness, particularly in high-risk or incurable patients. The market potential has expanded with the addition of lower-risk patient populations to the list of TAVR indications. The industry is anticipated to grow as more patient groups receive regulatory clearances. The increase in market presence is facilitated by collaboration between research groups and medical device firms.
- In November 2022, the $20 million series was an investment for Nyra Medical Inc., a medical device startup creating a new transcatheter mitral valve replacement method. Together with other investors, Broadview Ventures, Epidarex Capital, the Georgia research alliance venture fund, Vensana Capital, and a sizable international medical equipment business co-led the round.
- In February 2022, the largest manufacturer of medical devices in the world, Medtronic, plans to invest tens of millions of US dollars to construct core production lines for cardiovascular devices in Shanghai's free trade zone's Lingang area. The project's first phase will cost roughly $47.2 million, as disclosed to Yicai Global by the Minneapolis and Dublin-based company.
Regional Snapshot
North America holds the largest share of the transcatheter aortic valve replacement market. Older people are more likely to have aortic stenosis, and as North America's population ages, there is an increasing need for TAVR procedures. Continuous developments in TAVR technology, such as enhanced distribution methods, imaging methods, and valve designs, have helped TAVR become more widely used. Regulatory clearances and health authority cooperation greatly aid in the acceptance of the TAVR technique.
Several TAVR devices got FDA approval as of 2022, which fueled market expansion. Research and clinical trials were being carried out to assess the safety and effectiveness of TAVR in various patient populations and to broaden its use. North America has several key players in the Transcatheter Aortic Valve Replacement Market, including Abbott, Boston Scientific, Bracco Medical Technologies, Edwards Lifesciences, JC Medical, Inc., Jena Valve Technology, Inc., Medtronic, and Meril.
Globally, TAVR is becoming increasingly popular as a less intrusive substitute for conventional surgical aortic valve replacement. The market is growing due to factors like an aging population, aortic valve stenosis becoming more common, and technological developments. To guarantee safety and effectiveness, Health Canada is a critical player in regulating medical devices, including those connected to TAVR. Ongoing clinical trials and research in the field of TAVR may impact market dynamics. Adoption of TAVR operations might be influenced by public and private healthcare systems' reimbursement policies and coverage. Competitive dynamics, such as the introduction of new competitors and advances in technology, can impact market trends.
Changes in healthcare policy may impact the acceptance and accessibility of TAVR. Reimbursement rules and availability to TAVR operations may differ in the United States. Ongoing research and clinical studies aim to enhance the device's design, improve TAVR methods, and broaden the list of patients who qualify for the treatment. With multiple significant companies in the medical device sector, the TAVR market in the United States is competitive. The prevalence of aortic valve stenosis has increased in the United States due to the aging population. The FDA in the United States has granted regulatory approval for TAVR devices. Several manufacturers sell TAVR devices, and research is still being done.
- In May 2023, Medtronic, a pioneer in healthcare technology worldwide, plans to increase its presence in Hyderabad by investing Rs. 3,000 cr. Or around $ 350 million in its research and development center. This site now houses the company's largest facility outside the United States. The proposed investment is part of the company's global R&D-led innovation and growth strategy, and it will expand upon the initial $160 million investment announced for the Medtronic engineering and innovation center.
- In March 2023, its stakes in food and soft drink industries amounted to around $1.05 million, according to a freedom of information request. Additionally, the GMC invested more than Euro 1.2 million in pharmaceutical businesses, such as Novo Nordisk, AstraZeneca, Merck, and Roche; more than Euro 470,000 in private insurance or healthcare providers, such as UnitedHealth Group and Human Health; and more than Euro 1.3 million in companies that make medical devices.
Transcatheter Aortic Valve Replacement Market Report Scope
| Report Coverage | Details |
| Market Revenue in 2023 | USD 5.76 Billion |
| Projected Forecast Revenue by 2032 | USD 10.77 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 7.2% |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Clinical evidence and positive outcomes
Initially, patients deemed high-risk or inoperable and unsuitable candidates for conventional surgical aortic valve replacement were the primary recipients of TAVR procedures. TAVR services were then extended to low- and intermediate-risk patient groups later. Data from multiple trials, such as the Core valve high-risk trial and the PARTNER trials, with long-term follow-up, have demonstrated the long-term benefits of TAVR in terms of quality of life, symptom alleviation, and mortality. Modern technology, like the SAPIEN 3 and Evolute PRO valves, has been linked to better results and fewer issues.
Reimbursement policies
Private insurance provider reimbursement is another critical factor. Insurance companies and healthcare providers may negotiate pricing, and insurance policies may contain requirements. Payment frequently depends on particular standards or regulations that establish what constitutes a medically essential procedure. Formal health technology evaluation procedures, such as TAVR, are used in some nations to assess novel medical innovations' clinical and financial viability. Healthcare providers must follow coding requirements to identify and bill for TAVR operations. Specific codes are required for these procedures.
Restraints
Cost implications
The cost of the valve, the catheterization supplies, and other medical equipment required for the surgery are often high upfront costs for TAVR surgeries. A significant portion of the total cost of the TAVR surgery is related to the transcatheter heart valve. The cost of different valves from different manufacturers may vary. Hospital-related expenditures, such as facility fees, personnel charges, and post-operative care, are also included in the cost of the TAVR operation. Economic factors, such as currency fluctuations and inflation rates, can impact the whole cost structure of medical devices, including TAVR systems.
Access and adoption disparities
As different countries and regions have other healthcare infrastructures, reimbursement systems, and regulatory approvals, access to TAVR operations may fluctuate accordingly. Variations in healthcare institutions and processes may impact the implementation of TAVR. The prices and reimbursement policies for TAVR operations may impact how accessible the technology is. There may be differences in access when the procedure's cost and the reimbursement rates don't match in certain situations. Since TAVR is a specialty surgery, the availability of qualified medical personnel who can execute and support these procedures may impact disparities in access.
Opportunities
Global market expansion
Aortic stenosis is increasing in frequency in older people. As the world's population ages, so does the condition's frequency. Enhancements in the design of valves, methods of distribution, and imaging technology improved patient outcomes and increased the number of eligible patients. The market was expanding thanks to regulatory bodies worldwide approving TAVR devices. A more comprehensive range of patients could now access TAVR operations thanks to regulatory permissions. The benefits of TAVR over traditional treatments were emphasized through educational programs and clinical research.
Competitive pricing strategies
Businesses can implement a value-based pricing strategy by matching the cost of TAVR procedures to the estimated benefits they deliver to patients, healthcare providers, and payers. Companies can cater to various client segments' distinct requirements and inclinations by customizing their pricing strategies according to market segmentation. Businesses can regularly perform competitive benchmarking to ensure that their prices are competitive with the market. The pricing strategy may include creating patient assistance programs or working with foundations to facilitate patient access.
Recent Developments
- In September 2022, the SAPIEN 3 Ultra Resilia valve, which combines the industry-leading SAPIEN 3 Ultra transcatheter aortic heart valve with Edwards groundbreaking RESILIA tissue technology, was introduced by Edwards Lifesciences. The U.S. Food and Drug Administration just approved the launch.
- In December 2022, Global healthcare giant Abbott introduced the Navitor. This minimally invasive transcatheter aortic valve implantation technology is now accessible in India for patients with severe aortic stenosis who are at high or extreme surgical risk.
Key Market Players
- Medtronic plc
- Abbott Laboratories, Inc.
- Boston Scientific Corporation
- Meril Life Sciences Pvt. Ltd., Inc.
- Edwards Lifesciences Corporation
- St. Jude Medical, Inc.
- JenaValve Technology, Inc.
- Bracco SpA
- Transcatheter Technologies GmbH
Market Segmentation
By Implantation Procedure
- Transfemoral
- Transapical
- Transaortic
- Stainless Steel
- Nitinol
- Cobalt Chromium
- Others
By Material
- Stainless Steel
- Nitinol
- Cobalt Chromium
- Others
By Mechanism
- Balloon-expanding Valve
- Self-expanding Valve
By End-Use
- Hospitals
- Ambulatory Surgical Centers
- Others
Buy this Research Report@ https://www.precedenceresearch.com/checkout/3364
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308