Rapid Microbiology Testing Market Size and Forecast 2025 to 2034
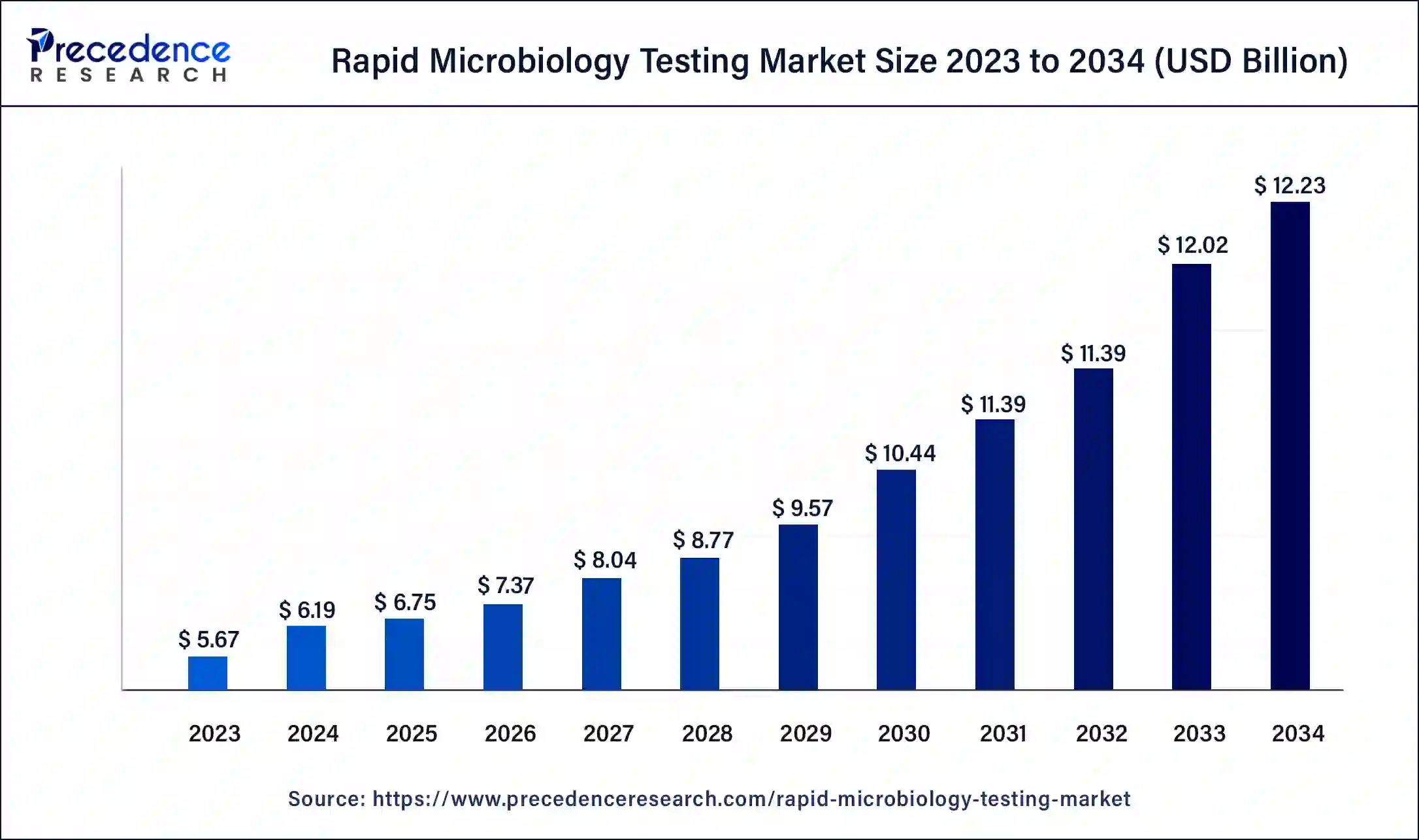
The global rapid microbiology testing market size was calculated at USD 6.19 billion in 2024 and is expected to reach around USD 12.23 billion by 2034, expanding at a CAGR of 7.05% from 2025 to 2034.
Rapid Microbiology Testing MarketKey Takeaways
- In terms of revenue, the rapid microbiology testing market is valued at $6.75 billion in 2025.
- It is projected to reach $12.23 billion by 2034.
- The rapid microbiology testing market is expected to grow at a CAGR of 7.05% from 2025 to 2034.
- North America led the global market with the highest market share of 38% in 2024.
- Asia-Pacific is expected to expand at the fastest CAGR during the forecast period.
- By Product, the Instruments segment has held the biggest revenue share of 43% in 2024.
- By Product, the consumables segment is projected to grow at a remarkable CAGR of 11.7% during the projected period.
- By Method, the growth-based rapid microbiology testing segment had the largest market share of 40.2% in 2024.
- By Method, the viability-based rapid microbiology testing segment is anticipated to grow at the fastest CAGR over the projected period.
- By Application, the clinical disease diagnostics segment has held the major revenue share of 25% in 2024.
- By Application, the research applications segment is projected to grow at a noreworthy CAGR of 12.7 % over the predicted period.
U.S. Rapid Microbiology Testing Market Size and Growth 2023-2032
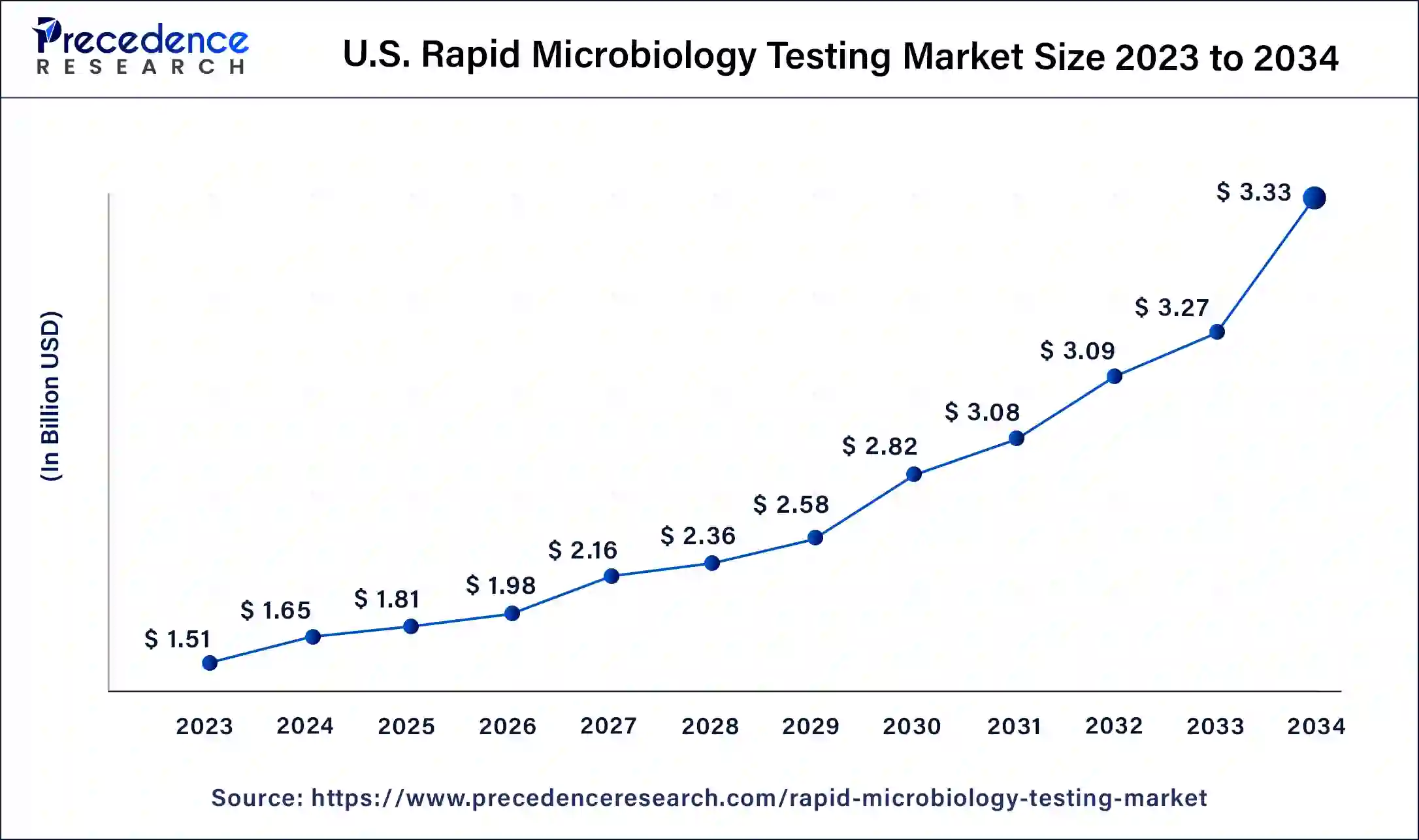
The U.S. rapid microbiology testing market size was valued at USD 1.65 billion in 2024 and is estimated to reach around USD 3.33 billion by 2034, growing at a CAGR of 7.27% from 2025 to 2034.
North America has held the largest revenue share 38% in 2024. North America dominates the rapid microbiology testing market due to several factors. The region's advanced healthcare infrastructure, stringent regulatory environment, and strong emphasis on food safety drive the demand for rapid microbiology testing. Additionally, the presence of major pharmaceutical and biotechnology companies, coupled with substantial R&D activities, spurs innovation in testing technologies.
Currently, North America is dominating the rapid microbiology testing market. The dominance imparts healthcare infrastructure, the largest amount of investment in the research and development area, and adoption and clear acceptance of advanced diagnostic technologies. The increased number of detected infectious diseases submissively supports the region to upskill in certain areas to serve the required facilities for the patients.
Moreover, the region's responsiveness to emerging healthcare challenges, like the COVID-19 pandemic, has accelerated the adoption of rapid testing solutions. These factors, along with a well-established market ecosystem, position North America as a major player in the rapid microbiology testing market.
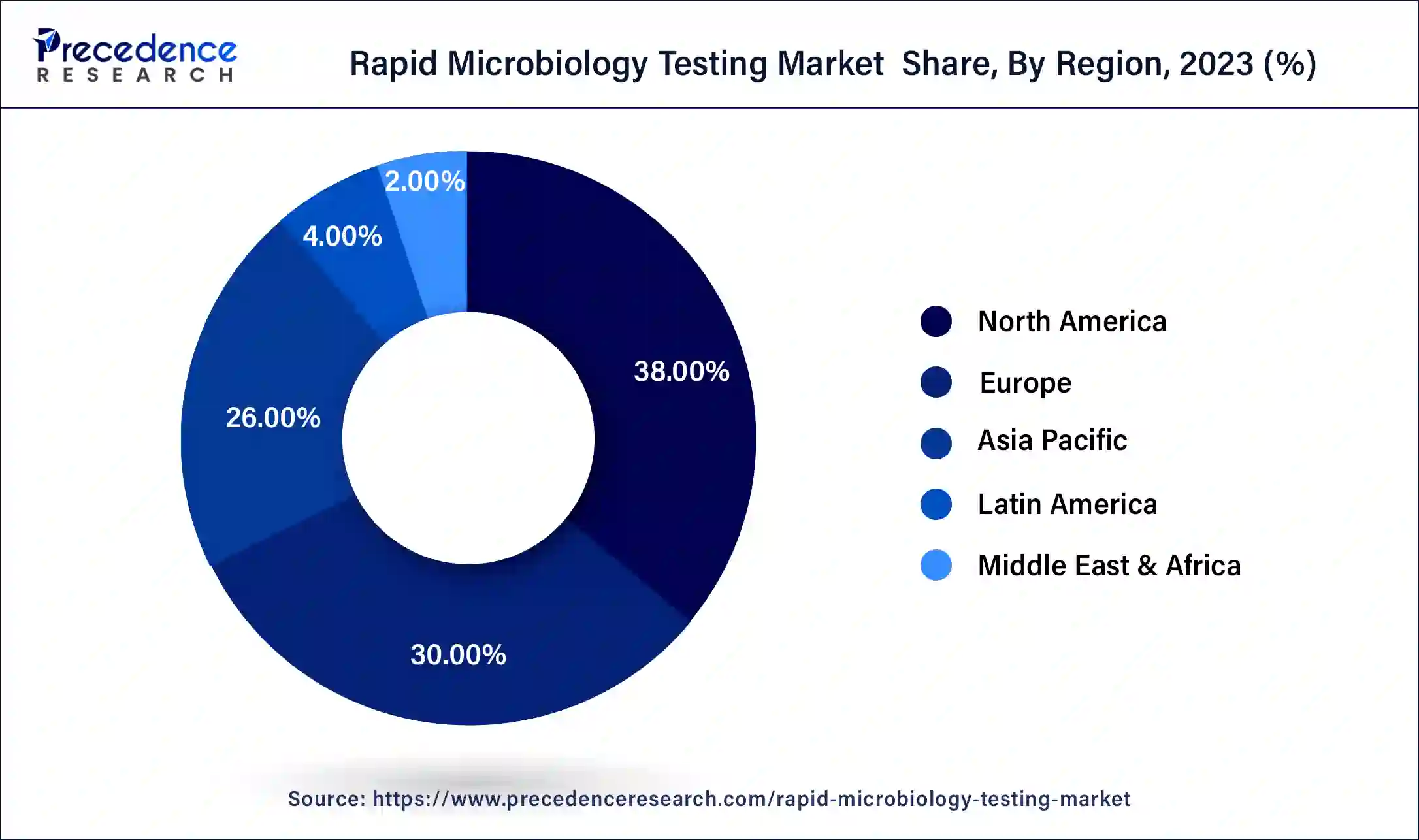
Asia-Pacific is estimated to observe the fastest expansion. The Asia-Pacific region holds significant growth in the rapid microbiology testing market due to several key factors. First, the region's substantial population and rapid urbanization have led to increased demand for food safety and healthcare, driving the adoption of rapid microbiology testing methods in these sectors. Second, the expanding pharmaceutical and biotechnology industries in countries like China and India require stringent microbiological quality control, further boosting the market. Lastly, government initiatives promoting technological advancements and healthcare infrastructure development contribute to the region's market dominance. These factors collectively position Asia-Pacific as a major growth leader in the rapid microbiology testing market.
Market Overview
The rapid microbiology testing market is a dynamic sector focused on providing fast and accurate methods for detecting and analyzing microorganisms in various industries, including pharmaceuticals, food and beverage, healthcare, and environmental monitoring. This market has witnessed significant growth due to the increasing demand for quicker and more efficient microbiological testing solutions to ensure product safety and quality. Key technologies driving this market include PCR, immunoassays, microbial enumeration, and molecular diagnostics. Factors such as stringent regulatory requirements, the need for rapid results, and the rising awareness of infection control have propelled the expansion of this market, with promising opportunities for future growth.
Rapid Microbiology Testing Market Growth Factors
The rapid microbiology testing market is a rapidly evolving sector dedicated to providing swift and reliable methods for the detection and analysis of microorganisms across diverse industries, including pharmaceuticals, food and beverages, healthcare, and environmental monitoring. This market has witnessed substantial expansion due to the escalating demand for more efficient microbiological testing solutions, ensuring product safety and quality. Key technologies like Polymerase Chain Reaction (PCR), immunoassays, microbial enumeration, and molecular diagnostics have played pivotal roles in driving this market.
One prominent trend in the rapid microbiology testing market is the continuous advancement of automation and robotics in testing processes. This automation not only enhances testing efficiency but also reduces the risk of human error. Additionally, the COVID-19 pandemic has underscored the importance of rapid and accurate microbiological testing, further catalyzing market growth. Increasing regulatory requirements and consumer demand for safe products are driving industries to adopt rapid microbiology testing methods to maintain compliance and build trust among their customers.
Despite its growth, the rapid microbiology testing market faces challenges related to the cost of implementing these advanced technologies, which can be prohibitive for smaller businesses. Moreover, ensuring the standardization and validation of rapid testing methods can be a complex and time-consuming process. Maintaining the accuracy and reliability of these methods also remains a challenge, as false negatives or positives can have significant consequences, especially in healthcare and food safety applications.
Amid these challenges, numerous opportunities abound in the rapid microbiology testing market. Companies that can develop cost-effective and easy-to-use testing solutions stand to gain a competitive edge. Expanding applications of rapid microbiology testing in emerging economies provide a vast untapped market for growth. Collaborations between technology providers, regulatory bodies, and industry players can help streamline validation processes and establish standardized protocols. Furthermore, continuous research and development efforts to improve the speed, sensitivity, and specificity of these tests will open doors to new business prospects.
In conclusion, the rapid microbiology testing market is driven by the imperative need for faster and more reliable microorganism detection methods across industries. While industry trends and growth drivers are propelling its expansion, challenges related to cost, validation, and accuracy persist. The market's future lies in capitalizing on emerging business opportunities, such as expanding into new geographic regions and developing innovative, cost-effective testing solutions to meet evolving industry demands.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 12.23 Billion |
| Market Size in 2025 | USD 6.75 Billion |
| Market Size in 2024 | USD 6.19 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 7.05% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product, Method, Applications, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Consumer demand for safety
The burgeoning consumer insistence on safety is a pivotal force propelling the expansion of the rapid microbiology testing market. Modern consumers are exceptionally attuned to the safety and quality aspects of products they consume, spanning pharmaceuticals, foodstuffs, beverages, and healthcare services. This heightened consciousness translates into a heightened demand for uncompromising safety standards.
The significance of rapid microbiology testing in meeting these consumer expectations cannot be overstated. Consumers now expect the products they utilize to be devoid of hazardous microorganisms and pathogens, and they gravitate toward goods that have been subjected to exhaustive testing to guarantee their safety. Consequently, industries such as food and beverage and pharmaceuticals are increasingly embracing rapid microbiology testing approaches to address these exacting demands.
Furthermore, in today's age of instantaneous communication and social media, news regarding safety lapses or contamination can disseminate with astonishing speed, causing severe damage to brand reputation and financial setbacks. Rapid microbiology testing empowers companies to proactively supervise and uphold product safety, thereby elevating consumer confidence and loyalty. Consequently, the market for rapid microbiology testing thrives as enterprises discern the imperative of aligning with consumer safety preferences and regulatory prerequisites.
Restraints
Validation and standardization
Validation and standardization represent significant restraints in the growth of the rapid microbiology testing market. Validation is a critical process to demonstrate that a testing method consistently produces reliable and accurate results. Achieving validation for rapid microbiology testing methods can be time-consuming, resource-intensive, and complex. This hurdle often deters companies from adopting these innovative approaches, especially in highly regulated industries like pharmaceuticals and healthcare. Standardization is equally challenging. Establishing universally accepted protocols and standards for rapid microbiology testing is an ongoing effort that involves collaboration between industry stakeholders and regulatory bodies.
The absence of standardized methods can lead to confusion and inconsistency in results, hindering wider adoption. Furthermore, regulatory compliance is contingent on validation and standardization. Companies must align their testing procedures with evolving regulatory requirements, adding another layer of complexity and uncertainty. In summary, the lack of streamlined validation and standardization processes can deter businesses from embracing rapid microbiology testing, limiting its growth potential and hampering its broader adoption in critical industries where safety and quality are paramount.
Opportunities
Pharmaceutical manufacturing
Pharmaceutical manufacturing is a catalyst for significant opportunities in the rapid microbiology testing market. The stringent regulatory requirements and the critical need for uncompromising product quality make rapid microbiology testing an essential component of pharmaceutical production. Opportunities abound in the development of advanced and specialized rapid testing solutions tailored to the pharmaceutical sector's unique demands, such as real-time monitoring of aseptic processes, microbial contamination control, and sterility testing.
Additionally, as pharmaceutical companies strive to expedite product development and time-to-market, rapid microbiology testing methods can streamline quality assurance processes, reducing production delays and costs. Collaborations between technology providers and pharmaceutical manufacturers are fostering innovation in this space, leading to the creation of more sophisticated and automated testing platforms. As the pharmaceutical industry continues to expand globally, the demand for robust and efficient rapid microbiology testing solutions is poised to grow, offering ample opportunities for market players to meet these evolving needs.
Impact of COVID-19
The COVID-19 pandemic has significantly impacted the rapid microbiology testing market. With the urgent need for widespread testing, particularly in healthcare settings, there has been a surge in demand for rapid testing solutions, such as PCR-based assays and point-of-care devices, to detect the virus. This has accelerated the development and adoption of rapid microbiology testing methods. However, disruptions in supply chains and regulatory challenges have also posed obstacles. Beyond the pandemic, the experience gained in deploying rapid testing has highlighted the importance of these technologies in healthcare and other industries, leading to continued growth and innovation in the rapid microbiology testing market.
Technological Advancement
Technological advancements in the rapid microbiology testing market feature instrumentation, automation, advanced testing methods, and point-of-care testing. The advanced testing methods include mass spectrometry, ATP bioluminescence, biosensors, and molecular diagnostics. Mass spectrometry is a technique that provides rapid identification of microorganisms according to their protein profiles. Biosensors and molecular diagnostics help in detecting the presence of microorganisms by identifying antigenic, metabolic, and genetic characteristics. ATP bioluminescence detects the presence of microorganisms with the help of an accurate measurement of ATP.
Point-of-care (POC) testing devices and portable diagnostics tools enable on-site testing, quick decision-making in emergency and critical areas covering mainly in hospitals. Instrumentation and automation reduce manual errors and streamline the testing process. Testing devices and miniature instruments enable accessibility and portability. It also allows point-of-care testing in various settings.
Product Insights
The Instruments sector has held 43% revenue share in 2024. The Instruments segment holds a major share in the rapid microbiology testing market due to its pivotal role in facilitating accurate and efficient testing processes. Instruments encompass a wide range of technologically advanced devices, including PCR machines, automated microbial identification systems, and microscopes, which offer speed, precision, and scalability. These instruments streamline sample preparation, analysis, and data management, making them indispensable in industries like healthcare and pharmaceuticals.
As the demand for rapid, reliable testing grows, the Instruments segment continues to dominate, driven by ongoing technological advancements and the need for high-throughput testing capabilities.
The consumables segment is anticipated to expand at a significant CAGR of 11.7% during the projected period. The consumables segment holds a significant share in the rapid microbiology testing market primarily because of its recurrent usage and necessity in testing processes. Consumables like test kits, reagents, and culture media are essential components for each test conducted, ensuring the accuracy and reliability of results.
As microbiological testing is routinely performed across industries such as healthcare, pharmaceuticals, and food production, the demand for consumables remains consistently high. Additionally, advancements in consumable technologies, such as pre-filled cartridges and disposable testing components, have further boosted their adoption, contributing to the segment's dominant market position.
Method Insights
The growth-based rapid microbiology testing sector had the highest market share of 40.2% in 2024 based on the method. The growth-based rapid microbiology testing segment holds a major share in the rapid microbiology testing market due to its established history and reliability. Growth-based methods, such as microbial culture and colony counting, have been the industry standard for decades. They offer a well-understood and trusted approach for detecting and quantifying microorganisms. While newer technologies like molecular diagnostics have gained traction, growth-based methods remain essential in various industries, including pharmaceuticals, food, and healthcare, as they provide vital data for quality control and compliance. Their proven track record and regulatory acceptance contribute to their continued dominance in the market.
The viability-based rapid microbiology testing is anticipated to expand at the fastest rate over the projected period. Viability-Based rapid microbiology testing holds a significant growth in the market due to its fundamental role in assessing microbial viability, which is critical in ensuring product safety and quality across various industries. This segment utilizes advanced techniques like ATP bioluminescence and impedance to provide rapid, real-time data on microorganism viability.
It enables swift decision-making, allowing for timely interventions when contamination is detected, reducing production downtime, and enhancing overall efficiency. Additionally, stringent regulatory requirements in industries like pharmaceuticals and food safety mandate viability assessment, further driving the demand for this segment's solutions and consolidating its market dominance growth.
Application Insights
The clinical disease diagnostics segment held the largest revenue share of 25% in 2024. The clinical disease diagnostics segment commands a substantial growth in the rapid microbiology testing market due to its pivotal role in diagnosing infectious diseases. The segment's prominence is driven by the increasing global burden of diseases like COVID-19, which necessitates rapid and accurate diagnostic methods for timely treatment and containment.
Furthermore, heightened healthcare awareness and the demand for point-of-care testing in clinical settings have boosted the adoption of rapid microbiology testing. As a result, this segment continues to dominate the market, with significant investments in research and development to enhance diagnostic accuracy and speed.
The research applications segment is anticipated to grow at a significantly faster rate, registering a CAGR of 12.7 % over the predicted period. The Research Applications segment commands significant growth in the rapid microbiology testing market due to its widespread use in various scientific research disciplines. Researchers heavily rely on rapid microbiology testing methods to expedite their studies and experiments, allowing for quicker analysis of microbial populations, facilitating drug discovery, and enhancing biotechnology research. These methods provide researchers with precise and timely results, enabling them to make informed decisions promptly.
Additionally, the continuous advancements in research technologies and the pursuit of scientific innovation contribute to the sustained dominance growth of the Research Applications segment in the market.
Rapid Microbiology Testing Market Companies
- Creative Diagnostics
- bioMérieux
- Synbiosis
- Thermo Fisher Scientific
- MERLIN
- Bio-Rad
- Danaher Corporation
- Merck Group
- Zhuhai DL Biotech.
- Hi-Media
- Liofilchem
- Bioanalyse
Recent Developments
- In January 2025, Rapid Infection Diagnostics Inc. launched a one-button solution for microbiology testing that provides pathogen ID and antibiotic sensitivity in 5 hours. It saves time by implementing a disruptive microbiology testing system.
- In November 2023, the cutting-edge microbiology innovations unleashed the power of microbiology. The molecular diagnostic, genomic approach, and automation will redefine the future of rapid microbiology testing laboratories.
- In November 2022, IDEXX made a strategic move by acquiring Tecta-PDS, an innovative Canadian company renowned for automating water microbiology testing processes, particularly for assessing parameters like E. coli and total coliforms. This acquisition significantly bolstered IDEXX's portfolio of water microbiology testing solutions, catering to both laboratory-based and in-field applications.
- During the same month, Archer Daniels Midland (ADM) expanded its microbiology capabilities with the inauguration of a new laboratory at its ADM Specialty Manufacturing facility in Decatur, Illinois. This expansion project not only doubled the size of the microbiology laboratory but also enhanced its testing capabilities, reflecting ADM's commitment to advanced microbiological analysis.
- In October 2020, Merck KGAA, based in Germany, entered into a collaboration with Mammoth Biosciences, a US-based company, with the aim of jointly developing, scaling up, and commercializing a CRISPR-based diagnostic test for SARS-CoV-2. This partnership leveraged the strengths of both companies in the pursuit of innovative diagnostic solutions.
- In September 2020, Bruker Corporation, headquartered in the US, expanded its offerings by acquiring Canopy Bioscience LLC, a US-based company specializing in targeted multi-omics and fluorescence-based imaging techniques. This strategic acquisition bolstered Bruker's capabilities in these areas, enhancing its position in the market.
- In July 2020, Biomérieux SA, a French company, introduced the Biofire mycoplasma test designed for the detection of mycoplasma contamination in biopharmaceutical products. This innovative test added to Biomérieux's portfolio of diagnostic solutions, addressing a critical need in the biopharmaceutical industry.
Segments Covered in the Report
By Product
- Instruments
- Reagents and Kits
- Consumables
By Method
- Growth-Based Rapid Microbiology Testing
- Cellular component-Based Rapid Microbiology Testing
- Nucleic Acid-Based Rapid Microbiology Testing
- Viability-Based Rapid Microbiology Testing
By Applications
- Clinical Disease Diagnostics
- Food & Beverage testing
- Pharmaceutical & Biological Drug Testing
- Environmental Testing
- Cosmetics and Personal Care Products Testing
- Research Applications
- Other Applications
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting