Rifaximin Market Size and Forecast 2025 to 2034
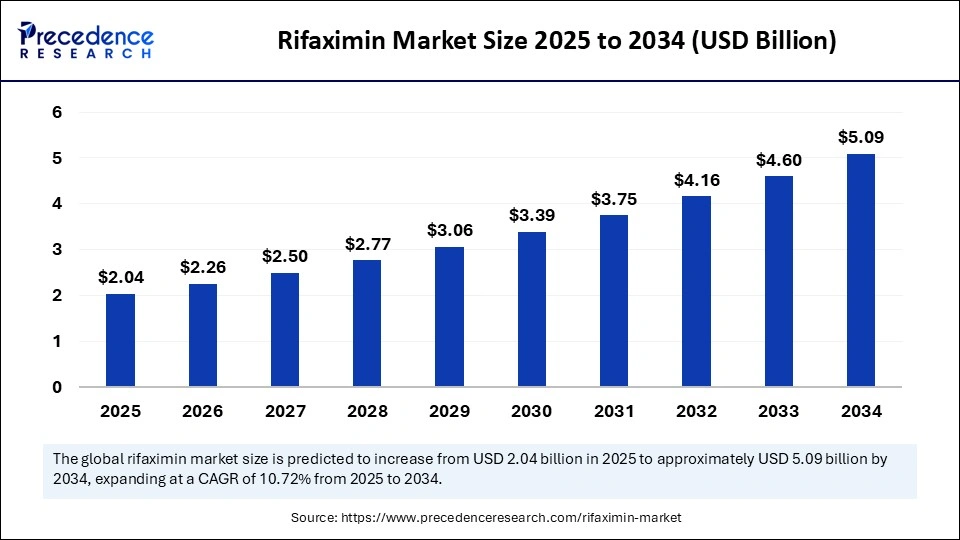
The global rifaximin market size accounted for USD 1.84 billion in 2024 and is predicted to increase from USD 2.04 billion in 2025 to approximately USD 5.09 billion by 2034, expanding at a CAGR of 10.72% from 2025 to 2034. The increased awareness about effective treatment options for gastrointestinal disease and demand for non-systemic antibiotics, like rifaximin driving the market.
Rifaximin Market Key Takeaways
- In terms of revenue, the global rifaximin market was valued at USD 1.84 billion in 2024.
- It is projected to reach USD 5.09 billion by 2034.
- The market is expected to grow at a CAGR of 10.72% from 2025 to 2034.
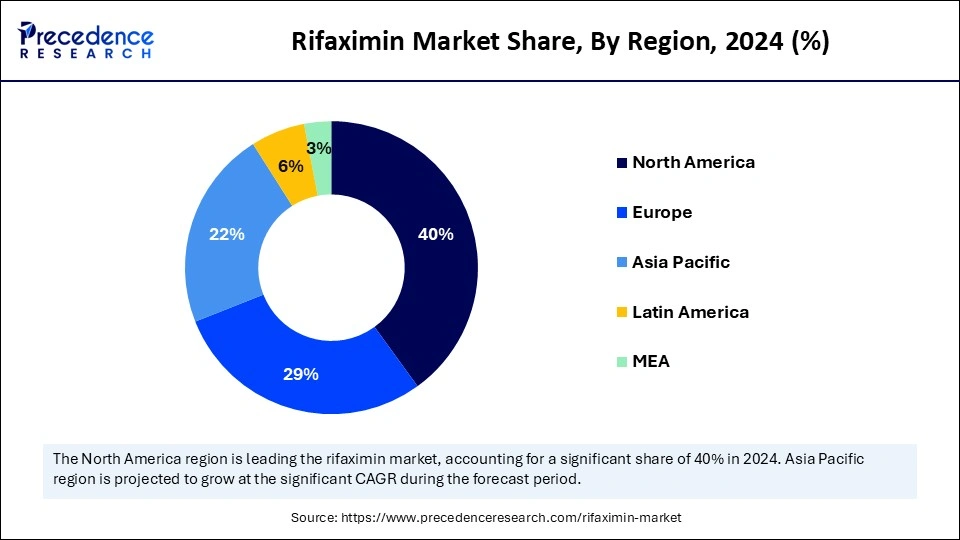
- North America dominated the global rifaximin market with the largest share of 40% in 2024.
- Asia Pacific is expected to grow at a significant CAGR from 20245 to 2034.
- By indication, the irritable bowel syndrome with diarrhea (IBS-D) segment contributed the largest market share of 45% in 2024.
- By indication, the hepatic encephalopathy (HE) segment is expected to grow at a notable CAGR between 2025 and 2034.
- By dosage form, the tablets segment captured the highest market share of 65% in 2024.
- By dosage form, the oral suspension segment will grow at a significant CAGR between 2025 and 2034.
- By distribution channel, the retail pharmacies segment generated the major market share of 50% in 2024.
- By distribution channel, the online pharmacies segment will grow at a significant CAGR between 2025 and 2034.
How is AI Playing a Role in Enhancing Rifaximin in the Global Market?
Artificial Intelligenceis playing a potential role in the production of efficient rifaximin. AI has proven its spectacular enhancements in pharmaceutical manufacturing processes related to Rifaximin, through ensuring polymorphic control, optimization of process synthesis, and automating quality control. The ability of AI to analyze a vast amount of data enables accurate and efficient predictions of optimal reaction conditions for the more efficient synthesis of rifaximin. AI use in monitoring the critical polymorphic properties of rifaximin for ensuring sustained drug quality and process streamlining from drug discovery, enhancing the readiness of rifaximin in the competitive market. Not only development but AI role in supply chain management and acceleration of regulatory submissions are enabling precise support for the market growth of rifaximin.
U.S. Rifaximin Market Size and Growth 2025 to 2034
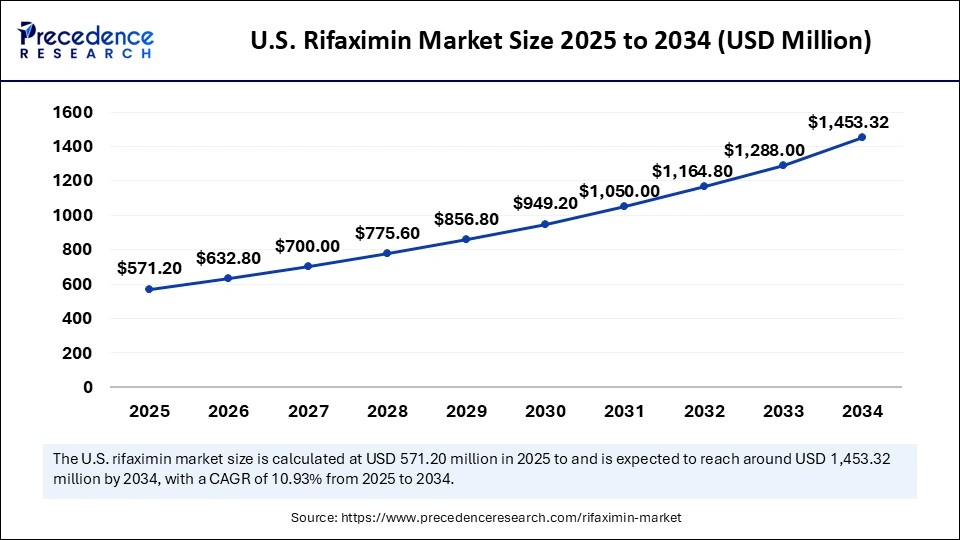
The U.S. rifaximin market size was exhibited at USD 515.20 million in 2024 and is projected to be worth around USD 1,453.32 million by 2034, growing at a CAGR of 10.93% from 2025 to 2034.
North America Rifaximin Market
North America dominates the global rifaximin market, driven by the increased prevalence of gastrointestinal disease and advanced healthcare infrastructure in the region. North America is the hub for a well-established healthcare infrastructure and high awareness about rifaximin's efficacy. The large patient population and robust healthcare system are driving the market. Additionally, the growing research and innovations in the rifaximin treatment are further adding to the market growth. Countries like the U.S., Canada, and Mexico are highly contributing to the region's dominance
The U.S. Market Trends
The U.S. is a major player in the regional market, growth driven by large gastrointestinal disorders and increased demands for effective antibiotic therapies, including rifaximin. According to the World Bank, the growing support for the demand for rifaximin and similar therapies is adding to the market growth. The growing FDA approvals for innovative research and developments, and novel rifaximin are contributing to the accessibility and availability of drugs in the U.S.
Asia Pacific Rifaximin Market
Asia Pacific, the fastest-growing region in the global market, contributes to the market due to expanding healthcare expenditure and rising disposable income. The improving healthcare infrastructure and government investments in local pharmaceutical manufacturing capabilities are leveraging the market growth. The growing aging population and availability of effective treatment options for gastrointestinal issues, including rifaximin, are contributing to the market dominance. Additionally, the increasing awareness about treatment options and demand for advanced treatment are contributing to the increasing demand for rifaximin in Asia.
Major Asia Pacific Countries Contributing to the Market
China and Japan are the key players in the regional rifaximin market, contributing to the growth due to the countries' improving healthcare infrastructure and growing interest in rifaximin. The strong existence of strong regulatory environments and government investments for local pharmaceutical manufacturing practices is contributing to the market growth. The growing aging population, increasing prevalence, rising disposable income, and expanding retail and online pharmacy networks are fostering the market's growth.
Market Overview
Rifaximin is a non-absorbable antibiotic used primarily for treating gastrointestinal disorders such as irritable bowel syndrome with diarrhea (IBS-D), hepatic encephalopathy, and travelers' diarrhea. It works by targeting gut bacteria without significant systemic absorption, reducing infection and inflammation while maintaining gut microbiota balance. The demand for the rifaximin 550 mg segment is high in the market due to its high efficacy in gastrointestinal disease treatments.However, the impact of U.S. tariffs on the global rifaximin market has led to an increased price of the drug. Trade policies and supply chain distribution have created significant challenges for U.S. and Chinese producers. Additionally, the primary patent protecting Xifaxan is projected to expire in 2028, which is expected to see the entry of a generic version of the drug in the market.
What are the Key Trends of the Rifaximin Market?
- Increased Gastrointestinal Disease Prevalence: The prevalence of gastrointestinal disease has increased, particularly traveler's diarrhea and irritable bowel syndrome with diarrhea, driving demand for rifaximin drugs.
- Awareness of Treatments: The awareness about treatment options for gastrointestinal disease has increased among healthcare providers and patients, driving the adoption of rifaximin.
- Demand for Non-systemic Antibiotics: The demand for non-systemic antibiotics like rifaximin has increased due to its good nature in terms of efficacy with minimal systemic side effects, making it a suitable option for patients with chronic gastrointestinal disease.
- Expanding Reimbursement Coverage: The government initiatives in promoting access to healthcare and increasing insurance coverage contribute to the rifaximin market.
- Focus on Microbiome: The growing awareness and focus on targeting gut bacteria that produce ammonia positions, driving research and developments in treatment options by using rifaximin.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 5.09 Billion |
| Market Size in 2025 | USD 2.04 Billion |
| Market Size in 2024 | USD 1.84 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 10.72% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Indication, Dosage Form, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Growing Aging Population
The prevalence of chronic conditions like irritable bowel syndrome (IBS) and hepatic encephalopathy has increased among the aging population. Aged people are mostly susceptible to gastrointestinal and liver diseases, driving the need for effective and non-systematic treatment options, including rifaximin. The awareness about gut health and the importance of healthy microbiome maintenance has increased, and so has increased demand for medications like rifaximin to help alter the gut flora. The growing government initiatives and healthcare insurance for significant facilities and treatment coverages are contributing to increasing hospitalizations of the aging population across the world. The adoption of rifaximin for aging populations is high in Europe, due to the region's vast elderly demographic.
Restraint
Fluctuation in Rifaximin Price
The price volatility and supply chain distribution are the major restraints for the global rifaximin market. The ongoing shift of trade policies, supply chain challenges, and increasing demand in different regions are influencing the price fluctuation of rifaximin. Price volatility limits the accessibility and affordability of rifaximin drugs, particularly for patients with chronic conditions and limited healthcare access. The U.S. tariff of 10% implementation has increased the import cost of rifaximin for pharmaceutical companies in certain regions, contributing to a price increase.
The U.S. has experienced a slight reduction in rifaximin prices due to rapid changes in trade policies in the country. Meanwhile, China has experienced a price increase due to strong demand for rifaximin within the country and supply chain issues.
Opportunity
Technological and Pharmaceutical Innovations
The ongoing technological and pharmaceutical innovations in rifaximin enable high-quality and efficient production of the active pharmaceutical ingredient (API). The ongoing innovations in enhancing drug delivery systems for better patient outcomes and overall efficiency, and compliance. The innovative rifaximin enables novel services, including predictive maintenance and repair. The growing focus on advancements in continuous manufacturing and nano-formulations is further adding to enhancements of rifaximin, contributing to increasing demand rates of gastrointestinal disorders and expanding healthcare access in the market.
Indication Insights
Which Indication Segment Dominates the Rifaximin Market?
The irritable bowel syndrome with diarrhea (IBS-D) segment dominated the market in 2024, due to the increased prevalence of IBS-D gastrointestinal disorder in a significant portion of the population. Irritable bowel syndrome with diarrhea (IBS-D) is becoming common in the elderly population, driving the need for effective rifaximin. The growing research for irritable bowel syndrome with diarrhea (IBS-D) by using rifaximin and its positive clinical trial outcomes are contributing to increasing its reliability and demand for specific treatments. Additionally, the FDA approvals for treatments for IBS-D for adults, due to enhancing symptoms of stool consistency and abdominal pain in a subset of patients, are contributing to the segment's growth.
The hepatic encephalopathy (HE) segment is the second-largest segment, leading the market due to increased hospitalization. The demand for effective treatments has increased due to growing complications of liver disease. The demand for rifaximin has increased due to its efficacy in reducing ammonia-producing gut bacteria. Rifaximin plays a crucial role in reducing the risk of HE recurrence and hospitalization. The increased prevalence of hepatic encephalopathy (HE) and limitations of other treatment solutions are driving demand for rifaximin.
Dosage Form Insights
What Made Tablets Dominate the Rifaximin Market in 2024?
In 2024, the tablets segment dominated the market due to its highly convenient nature. The tablet dosage form of rifaximin is highly efficient in treating various gastrointestinal disorders. The demand for tablets with two primary dosage strengths, including 200 mg and 550 mg, is high in the market. The 220 mg tablet is suitable for long-term maintenance and treatment of traveler's diarrhea and irritable bowel syndrome with diarrhea (IBS-D) conditions. The 200 mg rifaximin tablet is mostly recommended orally three times a day for three days. The 550 mg rifaximin tablet is highly reserved for high-intensity treatments. The hepatic encephalopathy condition is mostly cured by using a 550 mg rifaximin tablet. The dosage of a 550 mg tablet is mostly recommended for oral twice a day.
The oral suspension segment is expected to grow fastest over the forecast period, driven by its easy administration. The oral suspension of rifaximin is mostly recommended to certain patient groups, including elderly patients, pediatric patients, and patients with dysphagia. The oral suspension of rifaximin is typically in a concentration of 1 g/5ml or 2 g/5ml. The ease of administration, nature, and flexibility of oral suspension make them suitable for pediatric and elderly patients. Additionally, patients with dysphagia can get higher advantages of rifaximin by swallowing the formulation.
Distribution Channel Insights
Which Distribution Channel Segment Dominated the Market in 2024?
The retail pharmacies segment dominated the rifaximin market in 2024, due to easy and wide accessibility of rifaximin drugs in the retail pharmacies. Consumers with conditions of traveller's diarrhea and diarrhea-predominant irritable bowel syndrome (IBS-D) are the primary customers of the retail pharmacies. The demand for both the 200 mg and 550 mg rifaximin segments is leading the retail pharmacy areas. The wide accessibility of rifaximin in retail pharmacies makes them the primary point of contact for patients. Retail pharmacies offer expertise for counselling the patient on proper medication and dose intakes, making them a significant purchasing store for patients.
The online pharmacies segment is expected to grow rapidly over the forecast period, driven by a growing shift of consumers toward convenient shopping services. The increased adoption of e-commerce platforms and their offering of convenient ways for purchasing rifaximin, fueling the segment. The growing consumer preference for convenient and discreet purchasing options is shifting consumers toward online pharmacies. Additionally, the availability of home delivery services through online pharmacies makes it advantageous for patients with chronic conditions like IBS-D.
Value Chain Analysis
- R&D
The R&D in rifaximin is focusing on the discovery of novel polymorphs and exploring the efficacy of the drug for new indications for both FDA-approved and off-label, are increase the effectiveness of the drug.
Key Players: Salix Pharmaceuticals, AbbVie Inc., Fresenius Kabi AG, and Alfasigma S.p.A.
- Distribution to Hospitals, Pharmacies
Hospitals and pharmacies play a crucial role in initial prescription and inpatient management relevant to rifaximin. The direct distribution through the pharmacy community on behalf of LHUs.
Key Players: AbbVie Inc., Alfasigma S.p.A., Mylan N.V., and Salix Pharmaceuticals.
- Patient Support and Services
The significant patient support and services facilities, including Xifazan saving card, eVoucher, and patient assistance programs, are contributing to patient awareness and accessibility. The XIFAXAN is gaining traction due to its instant copay saving card to activate by calling 1-866-XIFAXAN.
Key Players: Salix Pharmaceuticals, Cleveland Clinic & Mayo Clinic, Apollo Hospitals, and MedlinePlus
Rifaximin Market Companies

- Alfasigma S.p.A.
- Salix Pharmaceuticals, Inc.
- F. Hoffmann-La Roche Ltd.
- Valeant Pharmaceuticals (Bausch Health Companies)
- Dr. Reddy's Laboratories
- Sun Pharmaceutical Industries Ltd.
- Macleods Pharmaceuticals Ltd.
- Glenmark Pharmaceuticals
- Zydus Cadila
- Lupin Limited
- Aurobindo Pharma Ltd.
- Cipla Ltd.
- Pfizer Inc.
- Novartis AG
- Sanofi
- Bayer AG
- Teva Pharmaceutical Industries Ltd.
- Mylan N.V.
- Hetero Drugs Ltd.
- Amneal Pharmaceuticals
Recent Developments
- In June 2025, the U.S. Food and Drug Administration (FDA) provided tentative approval for generic drugmaker Zydus Lifesciences' rifaximin tablets of 550 mg. The tablet is designed for use in the treatment of irritable bowel syndrome with diarrhoea (IBS-D) in adults. According to the company, the annual sales of rifaximin tablets reached $2672.9 million in the United States. (Source: https://www.thehindu.com)
- In January 2025, Bausch Health Companies Inc. and Salix Pharmaceuticals, its gastroenterology (GI) business, announced selection of the Centers for Medicare and Medicaid Services (CMS) for XIFAXAN (rifaximin) 550 mg tablets as one of the medicines for the nation's second round. These negotiations are part of the Inflation Reduction Act, with an applicability of an upfront price in 2027 of the Drug Price Negotiation programs. (Source: https://ir.bauschhealth.com)
Segment Covered in the Report
By Indication
- Irritable Bowel Syndrome (IBS-D) with Diarrhea
- Adult Patients
- Pediatric Patients
- Hepatic Encephalopathy (HE)
- Prevention
- Treatment
- Traveler's Diarrhea
- Prevention
- Treatment
- Others
- Small Intestinal Bacterial Overgrowth (SIBO)
- Infectious Diarrhea
By Dosage Form
- Tablets
- 200 mg
- 550 mg
- Oral Suspension
- 200 mg/5 mL
- Others
- Capsules
- Powder for Suspension
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting