Site Management Organization Market Size and Forecast 2025 to 2034
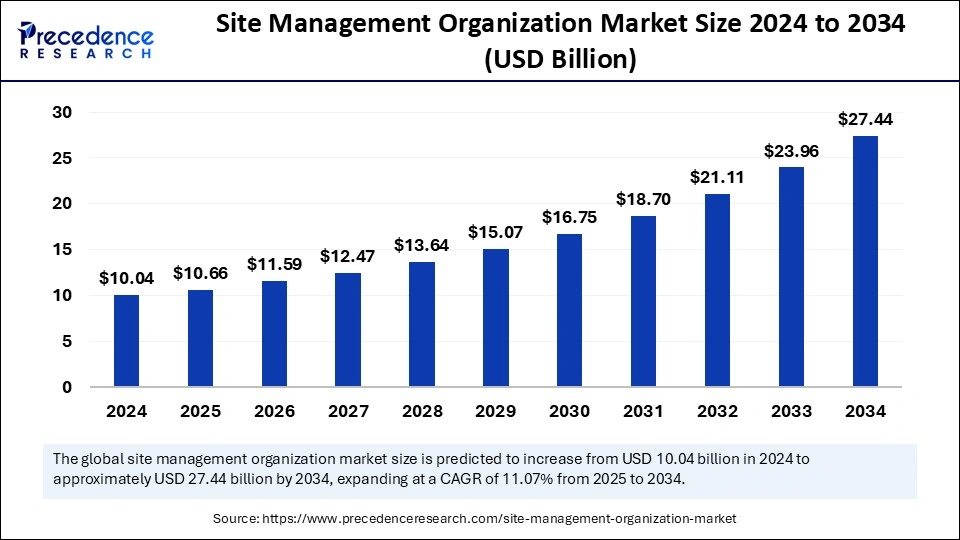
The global site management organization market size accounted for USD 10.04 billion in 2024 and is predicted to increase from USD 10.66 billion in 2025 to approximately USD 27.44 billion by 2034, expanding at a CAGR of 11.07% from 2025 to 2034. The growth of the site management organization market is driven by rising healthcare expenditures, increasing clinical trial activities, and increasing requirements for efficient site management support from pharmaceutical companies.
Site Management Organization Market Key Takeaways
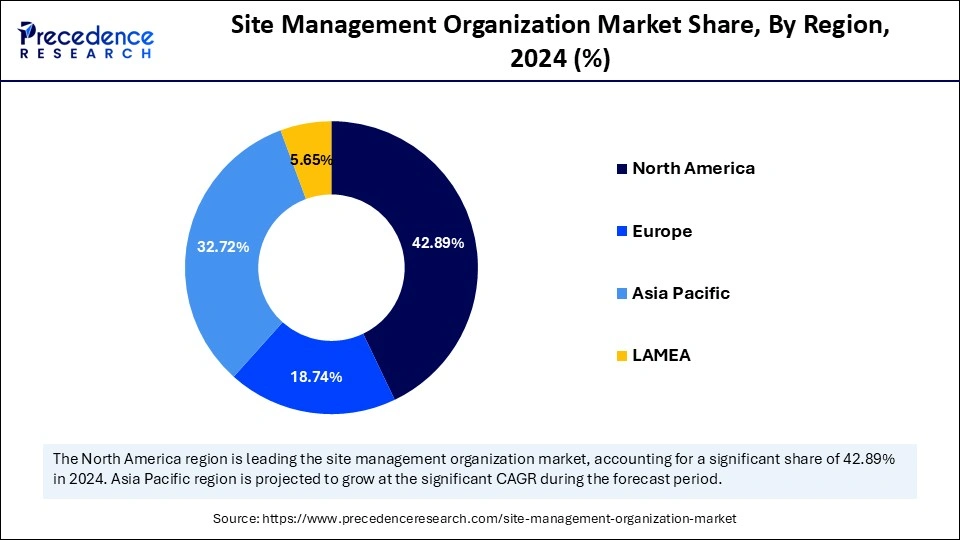
- North America dominated the market with the largest market share of 42.89% in 2024.
- Asia Pacific is anticipated to witness the fastest CAGR during the assessment years.
- By service type, the patient recruitment and retention segment held the biggest market share of 23.71% in 2024.
- By service type, the regulatory compliance segment is expected to grow at the fastest CAGR of 12% over the forecast period.
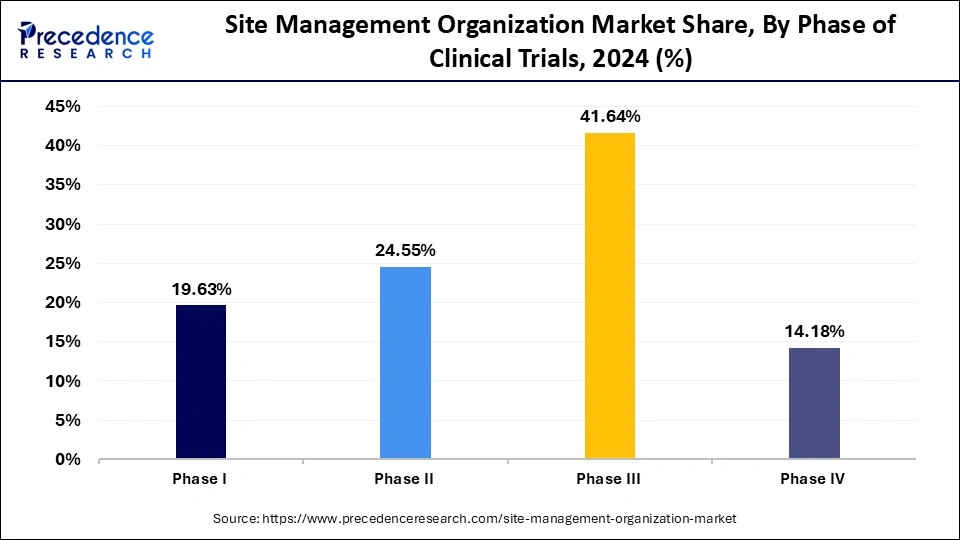
- By phase of clinical trials, the Phase III segment contributed the largest market share of 41.64% in 2024.
- By phase of clinical trials, the Phase II segment is expected to grow at a notable CAGR of 10% over the studied period.
- By therapeutic area, the oncology segment accounted for the highest market share of 33.30% in 2024.
- By therapeutic area, the neurology segment is projected to grow at a significant CAGR of 10.9% over the projection period.
- By end-user, the pharmaceutical companies segment generated the biggest market share of 39.26% in 2024.
- By end-user, the biotechnology companies segment is expected to grow at remarkable CAGR of 11.4% between 2025 and 2034.
Role of Artificial Intelligence in Site Management Organization Operation
Site management organizations can improve their operations through AI technologies. These technologies can enhance the efficiency of clinical trials by automating various tasks, such as patient enrollment and recruitment, data entry, and data analysis. Artificial Intelligence can analyze large datasets generated during clinical trials. This further helps in informed decision-making. AI also helps with site selection, quality improvement, and risk management.
- In October 2024, The Centers for Advanced Surgical Exploration (CASExGLOBAL) partnered with Clinical AI and iOR Partners to unveil first AI-powered site management organization.
U.S. Site Management Organization Market Size and Growth 2025 to 2034
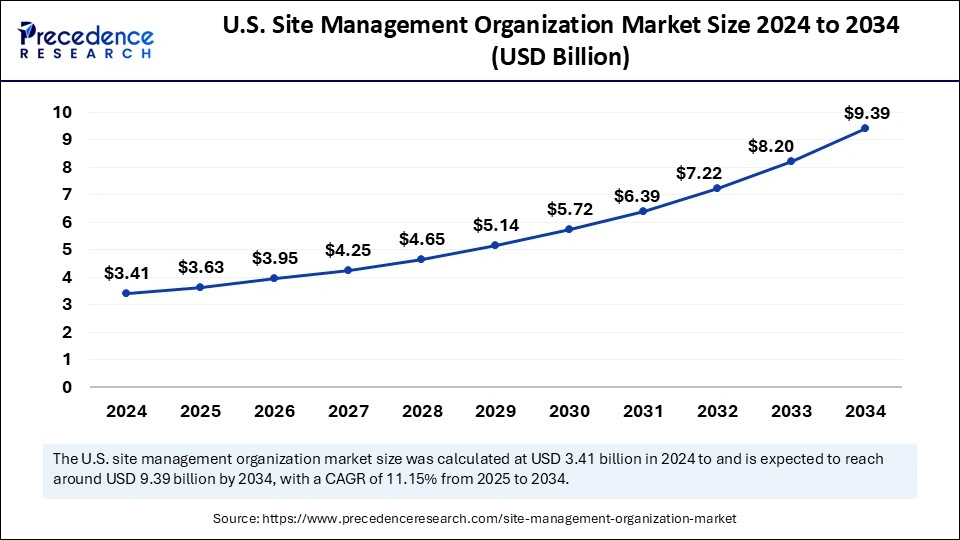
The U.S. site management organization market size was exhibited at USD 3.41 billion in 2024 and is projected to be worth around USD 9.39 billion by 2034, growing at a CAGR of 11.15% from 2025 to 2034.
North America's Stronghold on the Market
North America held the dominant share of the market in 2024. This is mainly due to its well-established research infrastructure. The region also boasts well-known research centers, educational institutions, and medical facilities. This led to an increased volume of clinical trials, boosting the demand for SMO services. Moreover, North American SMOs use innovative technologies for obtaining patient and site operations, leading to the successful execution of trials.
The U.S. is a major contributor to the North American site management organization market. The country is home to some of the world's leading pharmaceutical and biopharmaceutical companies that are actively engaged in clinical trials. The U.S. government supports clinical research activities. Stringent regulatory standards set by the U.S. FDA encourage research institutes and pharmaceutical companies to outsource clinical trials to SMOs.
Asia Pacific Site Management Organization Market Trends
Asia Pacific is expected to witness the fastest growth during the projected timeframe. The rising healthcare expenditure, stringent regulatory frameworks regarding pharmaceutical safety, and expansion of the pharmaceutical industry support regional market growth. The rising government investments in drug discovery and development lead to increased clinical trials. This, in turn, boosts the demand for SMO services.
China is expected to lead the market in the region in the foreseeable future. This is mainly due to the increasing clinical trial activities. Chinese SMOs are partnering with CROs to expand their footprints, contributing to market expansion.
- In March 2025, Novotech, an international CRO, and Acrostar, a Chinese company offering SMO services, entered into a partnership by signing an MOU with Kyungpook National University Hospital (KNUH). The partnership aimed to support clinical trials across KNUH's institutions, including the advanced clinical trials center, KNUH's main campus, and Chilgok KNUH in South Korea.
Europe Site Management Organization Market Trends
Europe is expected to observe notable growth soon. The growth of the European market is driven by increasing investments in research and clinical trials. Stringent regulations set by the European Union for clinical trials create immense opportunities for SMOs. In addition, the increasing focus on the development of novel therapeutics supports regional market growth.
China
China has merged as one of the most active hubs for global clinical trials, outpacing many traditional leaders in scale and speed. Its growth has been particularly strong in oncology and vaccines studies, supported by a vas diverse patient population and increasingly sophisticated research infrastructure. Regulatory reforms are reinforcing this trajectory, with authorities working to shorten clinical trial review timelines and align process more closely with international standards. Major academic and medical institutions, specially in mid to late stage research. The country is also gaining recognition for its innovative drug development, with a growing proportion of trial molecules originating locally. For site management organizations (SMOs), this environment demands capabilities in rapid patient recruitment, high volume site coordination, and robust compliance frameworks. The combination of regulatory acceleration, scientific capacity, and international integration makes China a pivotal market in the global SMO landscape.
India
India is rapidly positioning itself as a preferred location for clinical research, driven by a combination of regulatory modernization, cost advantages, and a diverse patient pool. Recent amendments to clinical trial rules have improved transparency, streamlines approval processes, and enhanced alignment with global standards. These changes are attracting greater interest from international sponsors and contract research organizations. Major players are expanding their operational footprint, adding trial sites across multiple states, and increasing investment in workforce development. In parallel, collaborations between local and global institutions are broadening the therapeutic focus of trials, particularly in areas such as oncology and chronic disease. For SMOs, India presents an expanding opportunity to deliver values through site identification, patient engagement strategies, digital monitoring solutions, and ongoing site training. The country's regulatory momentum and significant node in the global clinical research network.
Market Overview
Site management organizations (SMOs) serve as specialized third parties that provide support services to companies engaged in clinical trials. SMOs manage clinical trials efficiently through their supporting regulatory standards and quality control practices while optimizing operational efficiency. Complexity in clinical trials has made SMOs essential because their specialized expertise supports site operations and ensures trial performance success.
Drug discovery requirements have risen because of the increased prevalence of chronic diseases like cancer, diabetes, and cardiovascular disorders, thus leading to more clinical trials. The increasing production of pharmaceuticals, with new therapeutic drug clinical trials, boosts the need for SMO services to accelerate production.
Site Management Organization Market Growth Factors
- Increasing Drug Discovery Efforts: Pharmaceutical and biotechnology companies are continuously investing in drug discovery, leading to a significant rise in clinical trials. This, in turn, boosts the demand for SMO services. Site management organizations provide support to these companies to execute clinical trials.
- High Prevalence of Chronic Diseases: The rising number of cases of cancer, diabetes, and cardiovascular diseases is boosting the need for clinical trials focused on disease treatments. Outsourcing clinical trials to SMOs enables fast execution of these trials through their patient recruitment and regulatory compliance management services.
- Clinical Trial Outsourcing: Pharmaceutical and biotechnology companies increasingly use SMO services to manage trials because these services help reduce trial costs while providing effective solutions and maintaining regulatory requirements.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 27.44 Billion |
| Market Size in 2025 | USD 10.66 Billion |
| Market Size in 2024 | USD 10.04 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 11.07% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacfic |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Service Type, Phase of Clinical Trials, Therapeutic Area, End-user, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Growing Clinical Trial Complexity
Clinical trials are becoming increasingly complex, with more multi-site, multi-country studies, stricter regulatory requirements, and a growing focus on specialized therapeutic areas such as oncology and rare diseases. This complexity create demand for SMOs that can coordinate diverse sites, ensure regulatory compliance across jurisdictions and maintain data integrity in a fast paced environment. Sponsors are seeking partners who can not only manage administrative and operational burden but also accelerate site initiation and patient enrolment without compromising quality. The pressure to manage vast amounts of clinical data, combined with the need of adhere to evolving Good Clinical Practice GCP guidelines, position SMOs as critical enablers in ensuring that trials run smoothly, efficiently, and in line with international standards.
Pharmaceutical and biotechnology companies are increasingly conducting trials across diverse across patient populations, improve recruitments speed, and meet regulatory expectations for global submissions. This globalization trend fuels demand for SMOs with on-the-ground presence and local expertise, capable of bridging cultural, linguistic, and regulatory differences. SMOs that operates globally or have strong partnerships with regional organization can provide sponsors with integrated, scalable site management solutions. The ability to harmonize site operations across continents-while adapting to local patient engagement strategies and ethical oversight processes- has become a strong growth catalyst, especially for trials seeking simultaneous approvals in multiple high value markets.
Restraint
Regulatory Compliance and Data Security Concerns
Clinical trial operators face pressure to adhere to stringent regulations that govern clinical trials, ensure patient data security, and manage patient consent procedures. Changing regulatory requirements requires ongoing protocol revisions, practice updates, and staff training, which requires major financial support with specialized expertise. The widespread digitization of clinical trials pressurizes SMOs to establish strong security measures for protecting sensitive information, which can be challenging. Moreover, increasing competition from CROs restraints the growth of the site management organization market.
Despites the globalization of clinical research, regulatory processes remain highly fragmented. Each country and sometimes each region within a country can have distinct timelines, documentations requirement and ethical review procedures. This lack of harmonization increases operational complexity for SMOs, forcing them to allocate additional resources to navigate multiple systems. The variability can cause delay in site activation, extend trial timelines, and increase cost for both SMOs, the challenge is even greater, as they may lack the infrastructure or in country partnerships to effectively handle such regulatory diversity, limiting their ability to compete for large, multinational contracts.
The cost running clinical trial; sites is increasing due to factors such as inflation in labour expenses, higher technology licensing fees, and growing expectations for advance patient engagement tools. Additionally, maintaining compliance with stringent quality and safety standards requires continues investments in training, auditing and infrastructures updates. These rising costs can compress margins for SMOs particularly when working with sponsors that demand competitive pricing or when operating in markets where reimbursement timelines are slow. Without scale efficiencies or strategic cost controls, profitability can be difficult top maintain, especially for mid-tier and smaller SMOs.
Opportunity
Rising Global Healthcare Expenditure
Healthcare spending is steadily increasing, especially in emerging economies. Rising healthcare investment often translates into developing new therapeutics, leading to increased clinical trials. This creates opportunities for SMOs to tap into new marketplaces, especially emerging countries. Moreover, strategic partnerships between SMOs and CROs enable them to offer comprehensive services and expand their businesses worldwide.
Emerging markets in Asia, Latin America, and parts of Eastern Europe are experiencing rapid growth in trial activity driven by large patient pools, improving healthcare infrastructure and supportive government policies. These regions present fertile ground for SMOs to establish early presence, secure partnerships with local hospitals, and become preferred site management providers before market competition intensifies. Early entry also allows SMOs to develop deep local expertise, which is highly valued by sponsors seeking reliable recruitment and compliance in less familiar markets.
Technologies such as artificial intelligence, predictive analytics, and decentralized trial platforms are transforming site management operations. AI can optimize patient recruitments by identifying eligible participants from electronic health records, while remote monitoring tools can reduce the need for frequent on site visits lowering operational costs and improving efficiency. SMOs that invest in these capabilities can differentiate themselves by offering faster enrolment, enhanced data quality, and real time trial oversight. As sponsors increasingly prioritize speed and efficiency, digitally enabled SMOs will be well-positioned to capture a larger share of high value contracts in the coming years.
Service Type Insights
The patient recruitment & retention segment dominated the site management organization market with the largest share in 2024. With the increased volume of clinical trials, the need for patient recruitment has increased, since patient recruitment is a vital step to commence trial. Strategies for recruiting patients remain essential because they determine the attainment of target study populations and the proper duration of clinical trials. SMOs utilize digital platforms to enhance patient recruitment and retention. These services help pharmaceutical and biopharmaceutical companies to find eligible patients to keep trials on schedule.
Patient retention plays a vital role because leaving the participants delays trials as well as affects trial outcomes. Through optimized recruitment and retention processes, SMOs decrease the time and costs required for clinical trials. These services guarantee successful clinical trials by improving patient engagement and retention strategies.
The regulatory compliance segment is expected to grow at the fastest rate in the coming years. SMOs serve clinical research institutes by providing operational and administrative assistance, which maintains activities according to regional and international regulatory standards. Research institutes and clinical trial organizations must follow several regulations that regulate patient protection, clinical trial documentation, and privacy protection rules. SMOs with dedicated regulatory teams help guide through complex regulations, reducing penalties and trial delays.
Site Management Organization Market Revenue, By Service Type, 2022-2024 (USD Million)
| Service Type | 2022 | 2023 | 2024 |
| Site Selection | 1,384.2 | 1,474.0 | 1,578.4 |
| Patient Recruitment and Retention | 2,124.0 | 2,242.1 | 2,379.9 |
| Site Training and Support | 903.9 | 966.9 | 1,039.9 |
| Data Management | 1,224.1 | 1,281.2 | 1,348.3 |
| Regulatory Compliance | 858.8 | 926.2 | 1,004.2 |
| Monitoring Services | 1,767.9 | 1,859.5 | 1,966.7 |
| Project Management | 639.0 | 677.1 | 721.6 |
Phase of Clinical Trials Insights
The Phase III segment accounted for the largest share of the site management organization market in 2024. Site management organizations offer necessary services for conducting complex trials. Phase III trials are typically more complex and involve a larger patient population. Stringent regulations create complexities that boost the necessity for specialized services to handle trial operations. SMOs assist in navigating these complexities by providing necessary support.
The Phase II segment is expected to grow at a rapid growth rate during the forecast period. Phase II trials involve drug assessment to ensure safety and efficacy, requiring potential patients to evaluate the drug's efficiency and side effects. SMOs provide necessary services, including patient enrollment and regulatory compliance, to complete phase II trials successfully. Success in Phase II trials depends on on-site management to execute trials while effectively obtaining data because trials frequently use sophisticated research. The rising number of early-stage drug trials and escalating demand for targeted treatments support segmental growth.
Site Management Organization Market Revenue, By Phase of Clinical Trials, 2022-2024 (USD Million)
| Phase of Clinical Trials | 2022 | 2023 | 2024 |
| Phase I | 1,756.7 | 1,855.2 | 1,970.2 |
| Phase II | 2,193.0 | 2,318.4 | 2,464.6 |
| Phase III | 3,659.6 | 3,900.6 | 4,180.6 |
| Phase IV | 1,292.7 | 1,352.9 | 1,423.6 |
Therapeutic Area Insights
The oncology segment dominated the site management organization market. The increased cancer burden across the globe has boosted the need for novel therapeutics. According to the World Health Organization (WHO) projections, 30.2 million people will undergo cancer diagnoses in 2040, while cancer stands among the leading causes of mortality worldwide. Inactive lifestyle patterns increased cancer prevalence, driving the necessity for thorough research and clinical experiments.
SMOs specialized in oncology help in cancer research and trials because they provide operational support that enhances trial efficiency. Oncology trials tend to be complex and require many participants, requiring SMO services to navigate complexities and successfully complete research.
The neurology segment is expected to expand at the fastest rate over the studied period. The rising neuroscience research is a major factor boosting the growth of the segment. With the growing prevalence of neurological disorders, the development of novel therapeutics is increasing, leading to increased clinical trials for evaluating the efficacy of these therapies. Effective site management and patient retention remain paramount in neurological trials, boosting the need for specialized SMO services.
Site Management Organization Market Revenue, By Therapeutic Area, 2022-2024 (USD Million)
| Therapeutic Area | 2022 | 2023 | 2024 |
| Oncology | 2,897.6 | 3,103.8 | 3,342.9 |
| Cardiovascular | 1,782.1 | 1,884.5 | 2,003.9 |
| Neurology | 1,385.0 | 1,476.7 | 1,583.2 |
| Infectious Diseases | 1,117.3 | 1,166.8 | 1,225.1 |
| Metabolic Disorders | 953.2 | 1,003.0 | 1,061.1 |
| Others | 766.7 | 792.3 | 822.8 |
End-user Insights
The pharmaceutical companies segment accounted for the largest market share in 2024. Pharmaceutical companies often engage in research and development to discover new drugs, leading to a surge in clinical trials. This requires SMO services for the successful execution of clinical trials. Outsourcing clinical trials to SMOs enables pharmaceutical companies to focus on other core areas. SMOs help these companies navigate complexities during trials and getting drug approval process. SMOs provide essential solutions that help pharmaceutical companies adhere to strict regulations while successfully achieving trials. The rising investment by these companies in R&D further supports segmental growth.
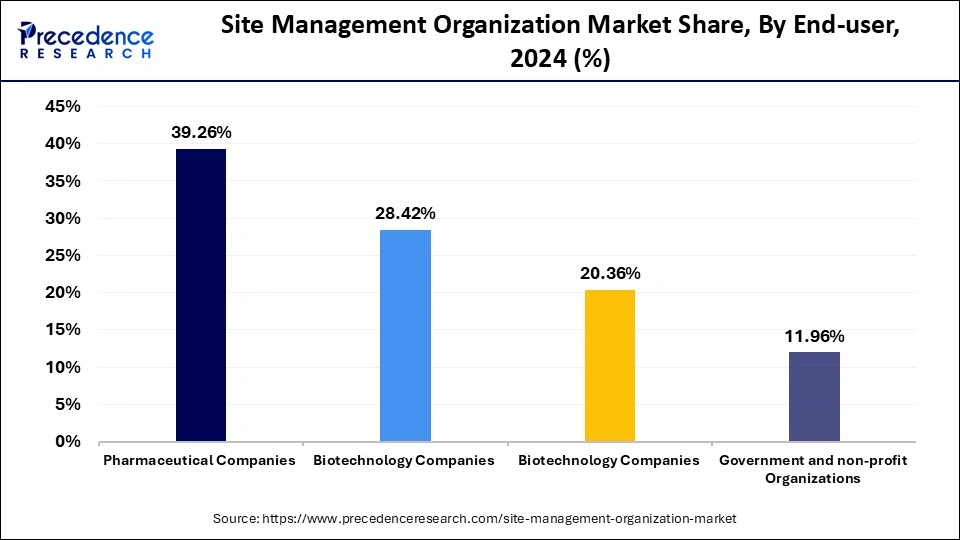
The biotechnology companies segment is anticipated to witness the fastest growth over the projection period. Biotechnology companies depend on SMOs for their key services, which include site management, patient recruitment, regulatory compliance, and data management to deliver efficient trials with regulatory standards. Due to the increasing demand for personalized medicine and rising innovations in biotechnology, the complexity of biotech clinical trials is growing, which boosts the need for SMOs. The characteristics of biotechnology products with novel approaches require specialized support from SMOs.
Site Management Organization Market Revenue, By End-User, 2022-2024 (USD Million)
| End-User | 2022 | 2023 | 2024 |
| Pharmaceutical Companies | 3,502.4 | 3,705.0 | 3,941.3 |
| Biotechnology Companies | 2,469.4 | 2,647.0 | 2,852.8 |
| Academic Institutions | 1,841.6 | 1,934.8 | 2,043.8 |
| Government and Non-Profit Organizations | 1,088.6 | 1,140.4 | 1,201.1 |
Site Management Organization Market Companies

- Novotech
- ERG Holding
- Apex Medical Research
- CMIC Group
- Tigermed
- FORMAT Medical Research
- EPSI
- AusTrials
- Beijing Aisimo Medical Science and Technology Co.
- Ltd
- MEDEX
- Ethic Co.
- ACTG-CRO
- CIDAL
- MPR Development Group
Recent Development
- In August 2025, In Pune, India following community complaints about mud and debris on the roads near Hinjewadi's Megapolis Township, the Maharashtra Industrial Development corporation(MIDC) launched a clean-up drive targeting construction site pollution. They mandated that vehicles existing construction sites must have covered loads and washed wheels to avoid depositing debris. Although such norms existed, enforcement was lax, residents highlighted safety risks, specially for two wheelers navigating slippery roads, and called for consistent action from builders and civic bodies. This local initiative has ignited calls across Pune, including areas like Baner, Kothrud, Karve Road, and Paud Road, urging stricter regulation and accountability in site transport protocols.
(Source- Hinjewadi road clean-up spurs citywide demand for stricter construction site transport rules | Pune News - Times of India ) - In August 2025, in the UK, construction activity plummeted at its steepest rate in over five years in July, with the S&P Global UK construction PMI dropping to 44.3 well below the neutral 50 threshold. This decline was most pronounced in the residential and civil engineering sectors, undermining the government's aim to build 1.5 million homes by 2029. The downturn was attributed to dwindling new orders, site delays, and shaky client confidence, prompting builders to reduce material purchases and staff. While some easing of material cost pressures and optimism about future pipelines were noted, the severity and pace of the construction signal continued challenges for site management organizations operating in the region.
(Source- UK construction activity falls by most in five years, survey shows)
Segments Covered in the Report
By Service Type
- Site selection
- Patient recruitment and retention
- Site training and support
- Data management
- Regulatory compliance
- Monitoring services
- Project management
By Phase of Clinical Trials
- Phase I
- Phase II
- Phase III
- Phase IV
By Therapeutic Area
- Oncology
- Cardiovascular
- Neurology
- Infectious diseases
- Metabolic disorders
- Others
By End-user
- Pharmaceutical companies
- Biotechnology companies
- Academic institutions
- Government & non-profit organizations
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting