Urodynamic Equipment and Consumables Market Size and Forecast 2025 to 2034
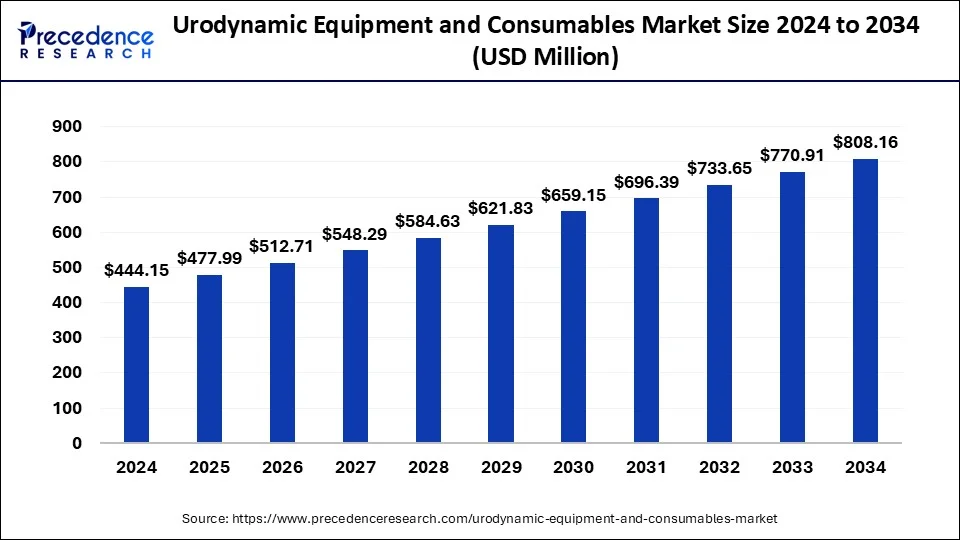
The global urodynamic equipment and consumables market size was USD 444.15 million in 2024, estimated at USD 477.99 million in 2025, and is anticipated to reach around USD 808.16 million by 2034, expanding at a CAGR of 6% from 2025 to 2034. The urodynamic equipment and consumables market is driven by the increasing prevalence of bladder and renal problems, especially in underdeveloped countries.
Urodynamic Equipment and Consumables Market Key Takeaways
- The global urodynamic equipment and consumables market was valued at USD 444.15 million in 2024.
- It is projected to reach USD 808.16 million by 2034.
- The urodynamic equipment and consumables market is expected to grow at a CAGR of 6% from 2025 to 2034.
- North America dominated the global market with the largest market share of 46.17% in 2024.
- By type, the uroflowmetry equipment segment contributed the highest market share of 25.3% in 2024.
- By type, the ambulatory urodynamic systems segment is the fastest growing during the forecast period.
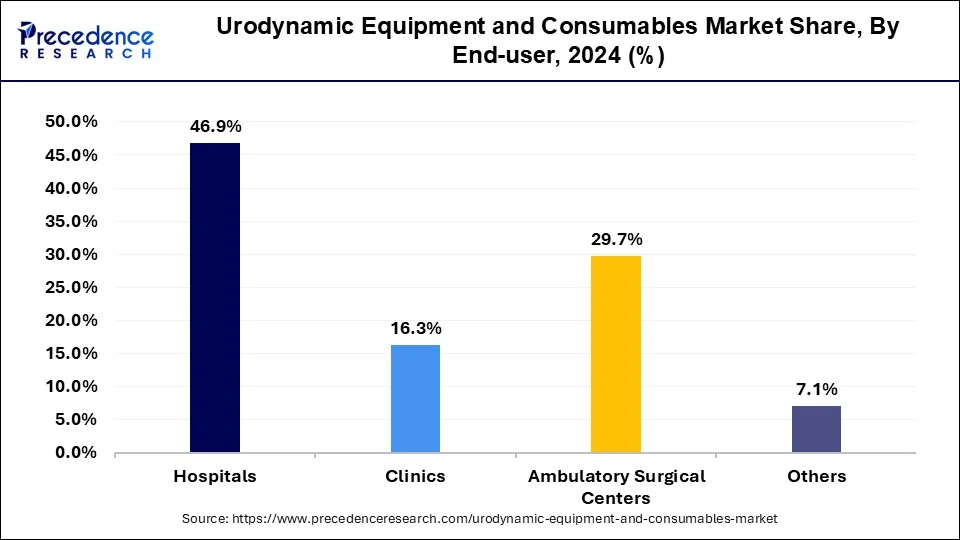
- By end-user, the hospitals segment captured the biggest market share of 46.9% in 2024.
U.S. Urodynamic Equipment and Consumables Market Size and Forecast 2025 to 2034
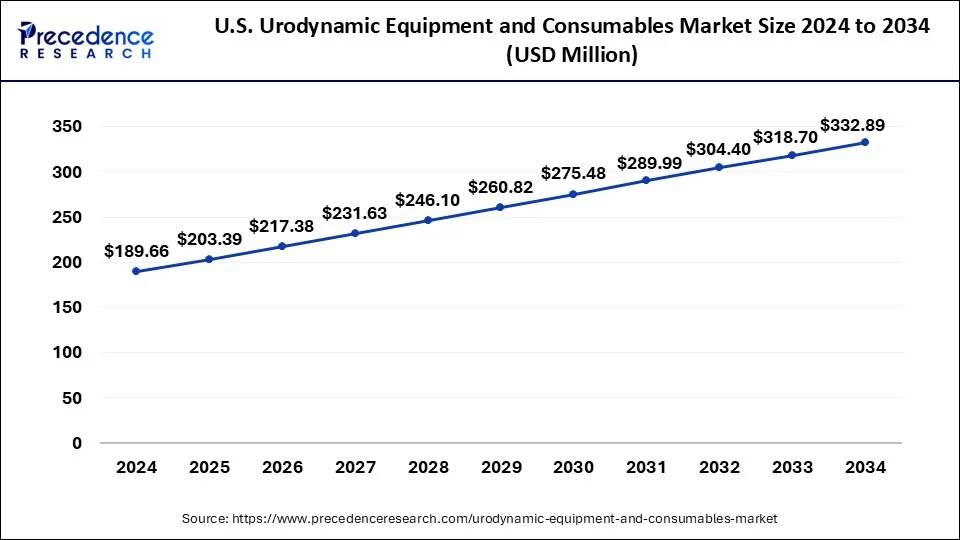
The U.S. urodynamic equipment and consumables market size was estimated at USD 189.66 million in 2024 and is projected to surpass around USD 332.89 million by 2034 at a CAGR of 5.60% from 2025 to 2034.

North America held the largest share in 2024 in the urodynamic equipment and consumables market and expected to sustain the position throughout the predicted timeframe. Most healthcare spending in North America goes toward treating and diagnosing urological disorders. Healthcare facilities are encouraged to invest in modern equipment and consumables by the region's high healthcare spending and favorable reimbursement regulations for urodynamic testing. The increased focus on early illness detection and preventative healthcare further fuels the need for urodynamic testing.
A well-established network of clinics, hospitals, and diagnostic facilities with cutting-edge medical technology, including urodynamic equipment, is present in the area. This makes it easier to acquire cutting-edge urological problem diagnosis and treatment methods, which increases demand for urodynamic tools and supplies.
- In February 2024, for children born with spina bifida, video urodynamic studies (UDS) are a standard component of therapy to monitor bladder function. These investigations have typically depended on fluoroscopy, which necessitates X-ray imaging. Instead, UVA Health Children's invented a novel method called contrast-enhanced voiding ultrasound, or ceVUS.
Asia-Pacific is the fastest growing urodynamic equipment and consumables market during the forecast period.Healthcare spending usually rises in the Asia-Pacific area as economies continue to thrive. This makes investing more heavily in cutting-edge medical technology like urodynamic apparatus possible. Urological problems are becoming more recognized among the public and medical experts. The need for urodynamic supplies and equipment increases due to the growing frequency of diagnosis and treatment. As minimally invasive procedures become more popular, the demand for urodynamic equipment will probably rise, enabling precise diagnosis.
Market Overview
The healthcare business segment focuses on supplies and equipment used in urodynamic testing and is known as the urodynamic equipment and consumables market. Urodynamic testing is a diagnostic technique that assesses the efficiency of urine storage and release in the bladder, urethra, and sphincters. It aids in the diagnosis of diseases like neurogenic bladder dysfunction, overactive bladder, and urine incontinence.
Urodynamic supplies and equipment allow medical professionals to carry out urodynamic tests precisely and effectively. This comprises a variety of tools, including catheters, electromyographs, uroflowmetry systems, cytometers, pressure transducers, and consumables, including tubing, sensors, and catheterization kits.
Urodynamic Equipment and Consumables Market Data and Statistics
- In December 2023, Bright Uro is a startup established in Irvine, California, that aims to improve clinic urodynamic testing efficiency, patient comfort, and physician accuracy. To assist in achieving these objectives, $23 million has been raised. Leading the Series A fundraising round was Laborie Medical Technologies, a producer of therapeutic and diagnostic products for urology.
- In August 2022, Health Canada has granted Voran Group Ventures Ltd. permission to market new efficacious claims for Bacoban Cleaner & Disinfectant, a Canadian-developed disinfectant solution. The updated label demonstrates Voran's dedication to providing Canadians with the best possible defence against the escalating risks of infectious illnesses.
Urodynamic Equipment and Consumables MarketGrowth Factors
- Diabetes and obesity are two lifestyle variables that raise the risk of urine incontinence, bladder dysfunction, etc., in the elderly population. Urodynamic testing is required as a result.
- Raised knowledge among the public on urological issues and available treatments, such as urodynamics.
- The development of ambulatory urodynamic systems that are more comfortable and less invasive is driving uptake.
- There is a need for equipment due to the growth of urology clinics and specialty hospitals in emerging nations.
- Better diagnosis results in better management of urological problems, which propels market expansion.
Market Scope
| Report Coverage | Details |
| Global Market Size in 2024 | USD 444.15 Million |
| Global Market Size in 2025 | USD 477.99Million |
| Global Market Size by 2034 | USD 808.16 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 6% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Consumables, End-user, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Driver
Increase in prevalence of lower urinary tract symptoms
Urinary symptoms that impact the bladder, urethra, and related tissues are lower urinary tract symptoms (LUTS). These symptoms might include urgency, frequency, nocturia, incontinence, and difficulty voiding. These symptoms can have a severe negative effect on a person's quality of life and are frequently a marker of underlying urological diseases such as overactive bladder (OAB), benign prostatic hyperplasia (BPH), urinary tract infections (UTIs), and neurological abnormalities that impair bladder function. Urodynamic testing processes are made more accessible by urodynamic equipment and consumables. This comprises disposable consumables like catheters, tubing, electrodes, and specialist equipment, including pressure transducers, uroflowmetry devices, cystometrography systems, catheters, and infusion pumps.
The development of more advanced urodynamic equipment with improved data collecting, analysis, and interpretation capabilities has been made possible by technological advancements, which have improved the precision and dependability of urodynamic testing techniques.
Restraint
High cost of equipment
Alternative diagnostic modalities like magnetic resonance imaging (MRI), ultrasound imaging, and non-invasive urodynamic procedures compete with urodynamic testing. Despite the possibility of cost reductions or other benefits, these substitutes might not give the same degree of thorough examination or diagnostic accuracy as conventional urodynamic testing. The expenditures of purchasing, maintaining, and training staff on urodynamic procedures may not be sufficiently covered by reimbursement rates in many healthcare systems. Consequently, the market growth may be limited if healthcare providers exhibit reluctance to invest in urodynamic equipment or restrict the provision of urodynamic services.
Opportunity
Government initiatives for incontinence treatment
Governments set aside large sums to enhance healthcare services, including facilities for diagnosing and treating ailments like incontinence. These expenditures pay for cutting-edge medical supplies, such as urodynamic testing apparatus and associated items. Governments frequently enact healthcare reforms and laws to enhance patient outcomes and lessen the total cost of healthcare. The need for urodynamic supplies and equipment will likely increase due to these reforms, which may include recommendations for using urodynamic testing for precise diagnosis and treatment planning in cases of urine incontinence.
Type Insights
The uroflowmetry equipment segment dominated the urodynamic equipment and consumables market in 2024. Since uroflowmetry is non-invasive, the urine system is not penetrated or instrumented throughout the process. Patients find this method less painful and daunting than other urodynamic examinations, such as cystometry or pressure flow studies. Because of this, medical professionals frequently choose to use uroflowmetry as their primary diagnostic method. Generally, it is cheaper than other urodynamic testing equipment. This makes it a desirable choice for medical establishments on a tight budget or looking for affordable diagnostic options. Uroflowmetry is also more cost-effective because it is non-invasive, which lowers related expenses like sedation, disposables, and recovery time.
The ambulatory urodynamic systems segment is the fastest growing in the urodynamic equipment and consumables market during the forecast period. Due to ambulatory urodynamic equipment, patients can conveniently undertake urodynamic testing outside of a clinical setting. Wearing these portable devices allows patients to continue their everyday activities, improving comfort and lowering anxiety related to traditional diagnostic methods used in clinics or hospitals. Wireless communication, downsizing, and sensor technologies have greatly enhanced the dependability and efficiency of ambulatory urodynamic systems. More thorough evaluations of urinary function are now possible thanks to these devices' ability to precisely measure various physiological characteristics, such as bladder pressure, urine flow rate, and detrusor muscle activity.
The electromyographs segment shows a significant growth in the urodynamic equipment and consumables market. Since electromyographs (EMG) can illuminate the neuromuscular mechanisms behind urine failure, researchers and clinicians increasingly use EMG-based urodynamic testing. Electromyography facilitates the evaluation of the patterns of muscle activity, reflexes, and the coordination of the many muscle groups involved in bladder control. Making treatment selections and diagnosing forms of urinary dysfunction depend heavily on this knowledge.
Urodynamic Equipment and Consumables Market Revenue, By Type 2022-2024 (USD Million)
| Type | 2022 | 2023 | 2024 |
| Uroflowmetry Equipment | 97.4 | 104.8 | 112.6 |
| Cystometer | 75.8 | 81.4 | 87.2 |
| Ambulatory Urodynamic Systems | 58.6 | 63.4 | 68.4 |
| Electromyographs | 46.1 | 50.1 | 54.2 |
| Video Urodynamic Systems | 37.9 | 40.9 | 44.0 |
| Urodynamic Consumables | 67.6 | 72.6 | 77.7 |
Consumables Insights
The urodynamics catheters segment dominated the urodynamic equipment and consumables market in 2024. Urodynamics catheters are essential components of urodynamic testing, a diagnostic procedure used to assess bladder and urinary tract function. These catheters are used to measure various parameters such as bladder pressure, urine flow rate, and sphincter muscle activity, providing valuable insights into urinary system disorders and dysfunctions. As urodynamic testing is a standard procedure for diagnosing conditions such as urinary incontinence, urinary retention, and overactive bladder, the demand for urodynamics catheters remains high among healthcare providers and urology clinics.
End-user Insights
The hospitals segment led the urodynamic equipment and consumables market in 2024. The segment is observed to witness a notable rate of growth during the forecast period. Hospitals are the primary healthcare providers for patients with urological conditions, such as urinary incontinence, overactive bladder, and urinary retention. As such, they have a high demand for urodynamic equipment and consumables to diagnose and manage these conditions effectively. Urodynamic tests, which assess bladder and urinary tract function, are routinely performed in hospital settings to aid in diagnosis and treatment planning.
Hospitals often invest in state-of-the-art medical equipment and facilities to deliver high-quality patient care. Urodynamic laboratories within hospitals are equipped with sophisticated urodynamic machines, pressure transducers, catheters, and other consumables necessary for performing a wide range of urodynamic tests, such as cystometry, pressure-flow studies, and electromyography. The availability of advanced facilities enhances the accuracy and reliability of urodynamic testing, making hospitals preferred providers for urology patients.
Urodynamic Equipment and Consumables Market Revenue, By End-user 2022-2024 (USD Billion)
| End-user | 2022 | 2023 | 2024 |
| Hospitals | 180.5 | 194.1 | 208.1 |
| Clinics | 62.2 | 67.1 | 72.3 |
| Ambulatory Surgical Centers | 112.9 | 122.3 | 132.1 |
| Others | 27.9 | 29.7 | 31.6 |
Urodynamic Equipment and Consumables Market Companies
- Laborie Medical Technologies
- The Prometheus Group
- Cooper Surgical
- Cook Medical
- Digitimer Ltd
- Verathon Inc
- Albyn Medical Ltd
- Ambu Inc
- MEDKONSULT Medical Technology
- SRS Medical
- Tic Medizintechnik
- HC Italia
- Stericom (P3 Medical)
- Santron Meditroni
Recent Developments
- In March 2024, Laborie Medical Technologies Corp., a leading provider of medical technology for diagnosis and treatment, revealed that it has invested in iO Urology. This venture-backed medical technology business created CarePath, an FDA-approved handheld uroflow device used in several urology clinics around the United States.
- In June 2022, Gemini Medical Technologies unveiled Atmos Air-Charged Urodynamics Catheters, the first patient-centric innovation in air-charged urodynamics catheters in ten years.
- In March 2022, In South Africa, Australia, and New Zealand, Sonoma Pharmaceuticals, Inc. introduced Microdox, a catheter and bladder rinse for urinary tract infections. A superoxidized solution, Microdox is based on the company's unique Microcyn Technology.
Segments Covered in the Report
By Type
- Uroflowmetry Equipment
- Cystometry
- Ambulatory Urodynamic Systems
- Electromyographs
- Video Urodynamic Systems
- Urodynamic Consumables
- Urodynamic Catheters
- Urodynamic Pumps and Transducer Sets
By Consumables
- Urodynamic Catheters
- Urodynamic Pumps
- Transducer Sets
By End-user
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting