What is the Vaccine Adjuvants Market Size?
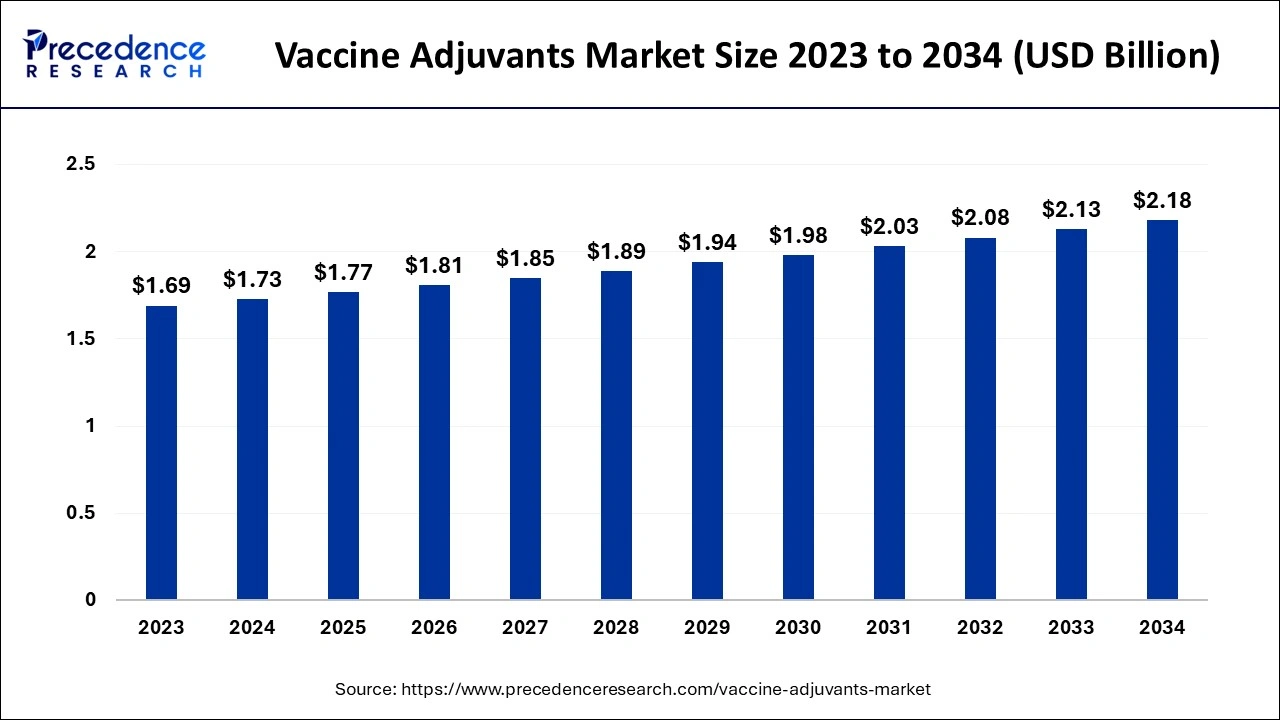
The global vaccine adjuvants market size is calculated at USD 1.77 billion in 2025 and is predicted to increase from USD 1.81 billion in 2026 to approximately USD 2.23 billion by 2035, expanding at a CAGR of 2.34% from 2026 to 2035.The increasing adoption of the vaccine adjuvants market is as it is an essential component of vaccines in enhancing both immune response and efficacy.
Vaccine Adjuvants Market Key Takeaways
- In terms of revenue, the vaccine adjuvants market is valued at $1.77 billion in 2025.
- It is projected to reach $2.23 billion by 2035.
- The vaccine adjuvants market is expected to grow at a CAGR of 2.34% from 2026 to 2035.
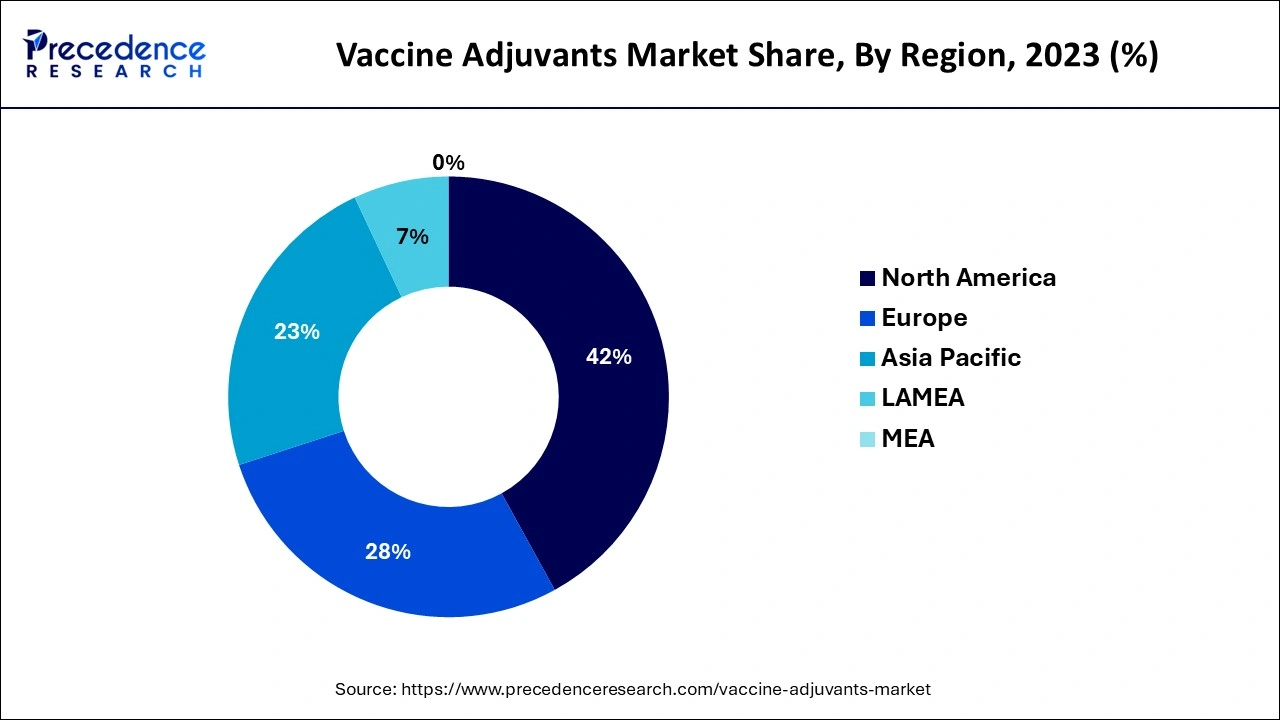
- North America dominated the global market with the largest market share of 42% in 2025.
- Middle East and Africa are anticipated to register significant growth during the forecast period.
- By type, the particulate segment captured the biggest market share in 2025.
- By type, the pathogen segment shows notable growth during the forecast period.
- By administration, the intramuscular segment contributed the highest market share of 34% in 2025.
- By administration, the intradermal segment will show notable growth during the forecast period.
- By application, the infectious disease segment generated the major market share in 2025.
- By application, the cancer segment is anticipated to grow at the fastest CAGR during the forecast period.
Market Overview
Adjuvants are components of vaccines that help to improve the proportion, and duration of the immune response to vaccination. Modernized adjuvants with improved properties including tailored humoral and cellular immune response, could provide a pathway for a new outbreak of innovative vaccines against existing and emerging pathogens. Currently, only a limited number of adjuvants are available for human use which are also licensed vaccines. Adjuvants have been used in vaccines for generations in the form of Aluminium salts, Aluminium Hydroxide, Aluminiun Phosphate, and Aluminon Potassium Sulphate. Nonetheless, the most effective of all is Aluminium salts.
How Artificial Intelligence (AI) is Changing the Vaccine Adjuvants Market?
The integration of artificial intelligence in the vaccine adjuvants market aids in the development of vaccine design, offering exceptional opportunities to accelerate the process. Algorithms such as machine learning and deep learning increase genomic data, protein structures, and immune systems interaction to predict antigenic epitopes, assess immunogenicity, and prioritize antigens for experimentation. AI in vaccine development emphasizes antigen selection, epitope prediction, adjuvant identification, and optimization strategies. The AI-driven point of view is used in the rational design of immunogens and the identification of new adjuvants with top-notch safety and efficacy outlines.
Market Key Trend
The vaccine adjuvant market is progressing rapidly, fuelled by breakthroughs in immunological science, heightened demand for high-efficacy vaccines, and the global push to combat both longstanding and emerging health threats. Adjuvants, crucial agents added to vaccines to boost the immune system's response, are playing an increasingly vital role in enhancing the performance of both conventional and next-generation vaccines, including those targeting chronic infections and oncology. The market's momentum is also driven by growing interest in personalised immunisation strategies and the pressing need for swift responses to global pandemics.
Vaccine Adjuvants Market Growth Factors
- Vaccine adjuvants have the potential to enhance immune potency to help the body produce a strong immune response in people receiving vaccines to protect against diseases.
- Vaccine adjuvants make vaccines more effective by improving the efficacy of the existing vaccines. Along with that, high efficacy using less antigen could lead to short supply and high cost.
- Reducing immunization scheduling is achieved with vaccine adjuvants reducing the amount of antigen per vaccine dose and the number of vaccination sessions. In some cases, they also increase the stability of the antigen components.
- It helps in the development of new vaccines for treating allergies, autoimmune diseases, and cancer.
Market Outlook
The vaccine adjuvant market is progressing rapidly, fuelled by breakthroughs in immunological science, heightened demand for high-efficacy vaccines, and the global push to combat both longstanding and emerging health threats.
The market is experiencing global expansion, driven by increasing demand for more effective vaccines against infectious and chronic diseases, government initiatives for pandemic preparedness, and advancements in adjuvant technologies.
Major investors and players in the vaccine adjuvants market include large pharmaceutical companies like GSK plc, Novavax, Inc., and CSL Limited. These companies invest heavily in R&D to create new adjuvant technologies.
The vaccine adjuvants market startup ecosystem is a dynamic environment focused on innovating substances that enhance vaccine efficacy and safety.
Market Scope
| Report Coverage | Details |
| Market Size by 2035 | USD 2.23Billion |
| Market Size in 2025 | USD 1.77 Billion |
| Market Size in 2026 | USD 1.81Billion |
| Market Growth Rate from 2026 to 2035 | CAGR of 2.34% |
| Largest Market | North America |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Segments Covered | Type, Application, Administrator, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Drivers
Modernization of vaccine
Vaccines containing new adjuvant formulations are gaining traction in the vaccine adjuvants market, which leads to reaching advanced development, and licensing stages, and providing new tools to fulfill previous unmet clinical requirements. The perspective of vaccine developers and regulators towards new vaccine adjuvants depends predominantly on the contribution of the adjuvant and the importance of the vaccine. The advancement in the biotechnology sector has brought significant growth toward modern vaccines based on rationally designed combined antigens containing highly purified components with suitable safety profiles. It is essential for the vaccine and adjuvant developers to fully utilize the mechanism of adjuvant mode of action avoid using non-specific components in adjuvant creation and develop extensive data packages on the safety, tolerability, and efficacy of adjuvanted vaccines.
Restraint
Strict regulation
The vaccine adjuvants market comprises stringent regulation which acts as a hindrance to the growth of the market. These requirements consist of rigorous testing for safety and effectiveness and to meet the quality standard with new and already existing vaccines. Some agencies and authorities such as CDSCO, ICMR, MoHFW, Department of Health Research, and ACIP (Advisory Committee on Immunization Practice) work together to ensure the vaccine approval process is properly regulated. Additionally regular reviewing of their processes and procedures to address any potential gaps.
Opportunity
Synthetic components developments
Currently, adjuvants are formulated mostly of natural components. The vaccine adjuvants market is continuously evolving with novel developments. Synthetic components are expected to be used in the future. However, the existing approved adjuvants will also continue to be used as valuable assets. Synthetic molecules have the potential to become more effective activators. These molecules can be produced in bulk at a low cost. Before replacing natural components with synthetic ones, there will be concerns about safety and protective efficacy for the clinical trial.
Segment Insights
Type Insights
The particulate segment captured the biggest share of the vaccine adjuvants market in 2025. The dominance of this segment is observed due to the immune system stimulation as it induces inflammatory responses. Several nanometers- to micrometer-sized particulates, such as particle matter 2.5 (PM2.5), sand dust, and diesel particles, cause pulmonary inflammation and allergic asthma. In contrast, these nanometer to micrometer-size particulates are utilized in the form of aluminum salts in vaccine adjuvants to enhance antibody response in humans and animals.
The pathogen segment shows notable growth in the vaccine adjuvants market during the forecast period. The expansion of this segment is experienced owing to vaccine adjuvants derived from pathogens such as bacterial or viral particles within the adjuvant which stimulate innate immune response, segmenting antigen presentation, and production of antibodies. This possesses the ability to mimic infectious agents while maintaining safety, offering a valuable asset in vaccine production
Administration Insights
The intramuscular segment contributed the highest share in the vaccine adjuvants market in 2025. The intramuscular route of administration is gaining traction due to its increasing popularity among patients as it provides a more sustained release of the vaccine and improves the efficiency of the vaccine. Intramuscular injections are absorbed faster than subcutaneous injections, as the muscle tissues contain greater blood supple compared to tissues just under the skin.
The intradermal segment will show notable growth in the vaccine adjuvants market during the forecast period. The growth of the intradermal route of administration is noticed as it has the longest absorption time of all parenteral routes. It is used for sensitivity tests, such as tuberculosis, allergy, and local anesthesia tests. Delivery of vaccine antigens to the dermis human skin results is more efficient than injection into the muscle or subcutaneous tissue, which leads to a reduction in the volumes of antigen.
Application Insights
The infectious disease segment contributed the highest share in the vaccine adjuvants market in 2025. The dominance of this segment is observed as it improves the vaccine efficiency by enhancing the immune response to a vaccine's antigens. As vaccines play a crucial role in limiting disease outbreaks and burdens, they are considered the best method for the long-term prevention of infectious diseases globally. The aging population is particularly at high risk due to the decline in immune function, which has potentially increased the risk of containing infectious diseases.
- According to WHO, the top ten primary deaths in low-income countries are associated with infectious diseases such as malaria, influenza, tuberculosis, and HIV.
The cancer segment is anticipated to grow at the fastest CAGR in the vaccine adjuvants market during the forecast period. The expansion of this segment can be credited to the utilization of adjuvants in preventative vaccines and therapeutic vaccines to enhance immune response but also uses personalized tumor antigens. A therapeutic cancer vaccine has the ability to stop the tumor from growing or spreading, destroy the cancer cell after surgery or radiation therapy, and prevent cancer from returning.
- In May 2024, scientists at Trinity College Dublin discovered a vaccine adjuvant called C100 which promotes potent antitumor immunity when injected directly into the tumor in an animal model.
Regional Insights
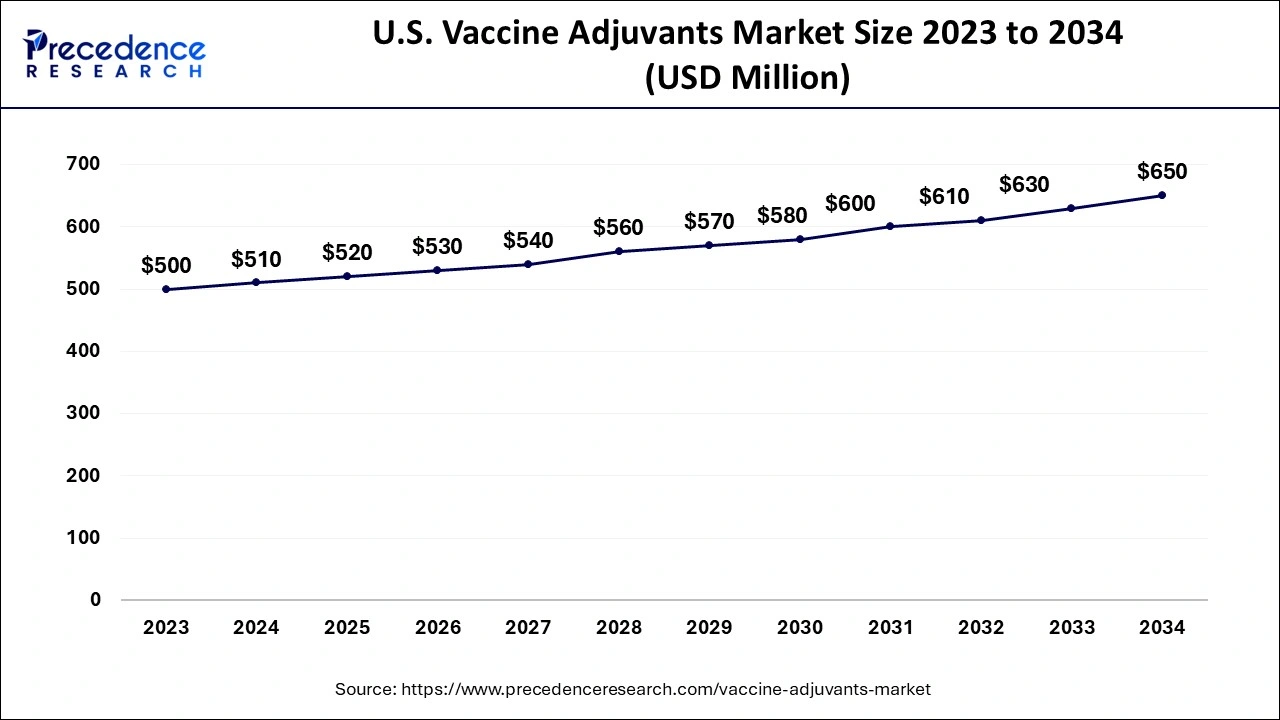
The U.S. vaccine adjuvants market size is exhibited at USD 520 million in 2025 and is projected to be worth around USD 670 million by 2035, growing at a CAGR of 2.23% from 2026 to 2035.
North America dominated the global vaccine adjuvants market in 2025. The dominance of this region is credited to the high commercial and research and development progress. A great investment by the U.S. government was made for the development, production, and purchase of vaccines. This public investment resulted in saving millions of lives and is crucial in the development of vaccine technology that also has the potential to address future pandemics and treat diseases beyond COVID-19.
- The government of the United States granted contracts totalling USD 31.9 billion for vaccine development during COVID-19, among which USD 337 million was invested in pre-pandemic.
North America stands at the forefront of the global vaccine adjuvant industry, with the United States and Canada driving considerable progress through well-established healthcare infrastructure and intensive research and development funding. Government bodies, particularly in the U.S., such as the Biomedical Advanced Research and Development Authority (BARDA), have actively financed and supported the advancement of innovative adjuvant formulations. Furthermore, the presence of leading biotech firms and collaborative ventures with research institutes has accelerated breakthroughs in adjuvant technology. Regulatory support and public health campaigns have further reinforced the adoption of advanced vaccine platforms in this region.
U.S. Vaccine Adjuvants Market Trends
Government bodies, in the U.S., such as the Biomedical Advanced Research and Development Authority (BARDA), have actively financed and supported the advancement of innovative adjuvant formulations. Furthermore, the presence of leading biotech firms and collaborative ventures with research institutes has accelerated breakthroughs in adjuvant technology.
Europe is expected to grow at a notable CAGR over the forecast period. The growth of the region can be attributed to increasing demand from vaccine development and rapid innovations in immunology research. The region also has a high concentration of vaccine manufacturers, especially in emerging countries.
Germany Vaccine Adjuvants Market Trends
The growth of the market in Germany can be driven by growing emphasis on technological innovations and advancements, especially in areas such as lipid nanoparticles and TLR agonists. Also, the rise of individualized medicine has driven the country's growth further, as it needs more tailored vaccine formulations to meet individual immunological needs.
Middle East and Africa are anticipated to register significant growth in the vaccine adjuvants market during the forecast period. The expansion of this region is observed due to the rapid increase in the number of infectious diseases, rapid technological advancement, and rising healthcare costs, contributing to fuelling the market growth. The issues are addressed through the improvement in vaccine and vaccine adjuvant technology.
In the Middle East and Africa, the vaccine adjuvant market is gradually strengthening due to a combination of healthcare modernisation, rising disease prevalence, and increased public-private partnerships. Countries like Saudi Arabia are investing in comprehensive immunisation strategies, while global organisations such as the WHO and Gavi are enhancing regional vaccine access. This growing support, along with national efforts to prevent infectious diseases through more potent and reliable vaccines, is helping the MEA region bridge the gap in advanced immunisation coverage. Efforts to introduce novel adjuvant-based vaccines for regional diseases such as pneumococcal infections and influenza are gaining traction, fostering market growth.
Saudi Arabia Vaccine Adjuvants Market Trends
Saudi Arabia is investing in comprehensive immunisation strategies, while global organisations such as the WHO and Gavi are enhancing regional vaccine access. This growing support, along with national efforts to prevent infectious diseases through more potent and reliable vaccines, is helping the MEA region bridge the gap in advanced immunisation coverage.
Vaccine Adjuvants Market Value Chain Analysis
This initial stage involves identifying and developing novel adjuvant candidates (e.g., TLR agonists, saponins, lipid nanoparticles) that can enhance immune responses.
This involves sourcing high-grade, often specialized, materials. Purity and sustainability (e.g., plant-based squalene) are increasingly important considerations.
This stage involves the large-scale production of the adjuvants, often necessitating specialized equipment and adherence to Good Manufacturing Practices (GMP).
The primary end-users include hospitals, clinics, and government immunization programs, with an increasing focus on meeting the needs of specific populations like the elderly or immunocompromised.
Vaccine Adjuvants Market Companies
Known for its proprietary CpG 1018, a synthetic TLR9 agonist used in HEPLISAV-B hepatitis B vaccine and licensed for use in several COVID-19 vaccines.
Offers adjuvants like AS01 and AS04 and provides adjuvanted vaccines such as the RSV vaccine Arexvy.

Other Major Key Players
- Agenus
- Merck KGaA
- InvivoGen
- CSL Limited
- Croda International PLC
- OZ Biosciences
- SEPPIC
- SPI Pharma
- Phibro Animal Health Corporation
- Sanofi
- Aphios Corporation
- Avanti Polar Lipids, Inc.
- Vertellus
Recent updates on vaccine adjuvants
Increased global immunization drives demand for adjuvants
- In March 2025, the market for vaccine adjuvants is growing quickly because of more immunization programs and pandemic preparedness efforts. In both preventative and therapeutic vaccines, adjuvants are now essential ingredients because they improve the immune response to vaccinations. To combat newly emerging and re-emerging infectious diseases, developing nations are increasing their use of adjuvanted vaccines. WHO-supported programs and government-sponsored immunization campaigns are increasing demand worldwide, especially for adjuvants that work with new vaccine platforms.
Innovation in adjuvant technologies enhances efficacy and safety
- By April 2025, Next-generation adjuvants like saponin-based (e. g. To improve vaccine performance, lipid nanoparticles, Matrix-M, and toll-like receptor (TLR) agonists are used. These developments are intended to support rapid production, enhance antigen stability, and lower dose requirements. Additionally, combination adjuvants are becoming more popular due to their capacity to stimulate both innate and adaptive immunity. To comply with clinical trial and regulatory requirements, manufacturers are investing in low-toxicity, scalable formulations.
Latest Announcements by Industry Leaders
- In October 2024, Freek Snieders, CEO, of SPI Pharma said, “This partnership between SPI Pharma and Inimmune represents a powerful synergy of strengths. By bringing together SPI Pharma's global commercial infrastructure with Inimmune's cutting-edge expertise in biotechnology, we are poised to accelerate innovation and bring transformative solutions to the market. Together, we will enhance our capabilities to improve patient outcomes worldwide.”
Recent Developments
- On 10 January 2025, the vaccine adjuvants market saw notable growth fueled by demand for better vaccine effectiveness. Innovations in adjuvant formulations, such as new particulate adjuvants and emulsions, were key drivers. These advancements aim to boost immune responses in vaccines targeting infectious diseases and cancer.
- On 18 February 2025, industry experts pointed to the increasing prevalence of infectious diseases like tuberculosis and hepatitis as major factors pushing the vaccine adjuvants market forward. The critical role of adjuvants in enhancing vaccine formulations has prompted more investment in related research and development.
- On 12 March 2025, regulatory agencies and healthcare leaders highlighted how adjuvants improve vaccine efficacy and help reduce the number of doses needed. By inducing stronger immune responses, adjuvants contribute to broader vaccine coverage and better public health outcomes.
- In May 2025, Andhra Pradesh Chief Minister N. Chandrababu Naidu launched Biovet, a company of the Bharat Biotech group, and its new vaccine against lumpy skin disease (LSD) in dairy cattle and buffaloes, the company announced in a statement.
- In October 2024, SPI Pharma, a global leader in biopharmaceutical excipient and adjuvant systems, and Inimmune, Corp., a fast-moving innovative biotechnology company, introduced their partnership to bring two adjuvant systems to the market. This partnership aims to launch a new adjuvant system at the World Vaccine Congress Europe, held in Barcelona.
- In December 2024, Novavax Inc., a global company advancing protein-based vaccines with its Matrix-M™ adjuvant, announced it had signed a definitive agreement to sell its manufacturing facility in Bohumil, Czech Republic to Nova Nordisk for USD 200 million.
Segments Covered in the Report
By Type
- Pathogen
- Adjuvant EMULSION
- Particulate
- Combination
- Others
By Application
- Infectious DISEASE
- Cancer
- Others
By Administrator
- Oral
- Intradermal
- Intranasal
- Intramuscular
- Others
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting