What is the Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Size?
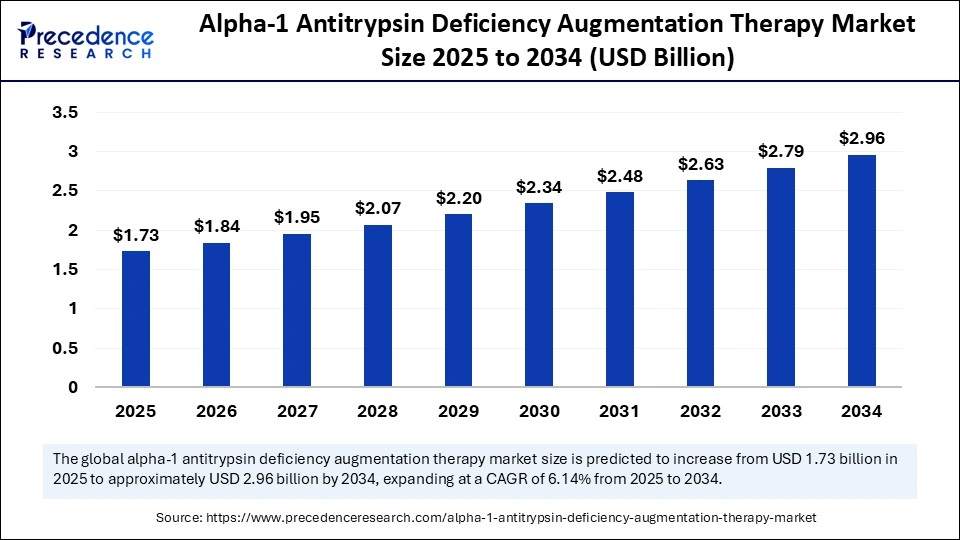
The global alpha-1 antitrypsin deficiency augmentation therapy market size accounted for USD 1.73 billion in 2025 and is predicted to increase from USD 1.84 billion in 2026 to approximately USD 2.96 billion by 2034, expanding at a CAGR of 6.14% from 2025 to 2034. The growth of the alpha-1 antitrypsin deficiency augmentation therapy market is driven by rising awareness of genetic diseases, increasing diagnosis rates, technological advancements in protein therapies, and growing demand for targeted treatments for genetic respiratory disorders.
Alpha-1 Antitrypsin Deficiency Augmentation Therapy MarketKey Takeaways
- The alpha-1 antitrypsin deficiency augmentation therapy market was valued at USD 1.63 billion in 2024.
- It is projected to reach USD 2.96 billion by 2034.
- The market is expected to grow at a CAGR of 6.14% from 2025 to 2034.
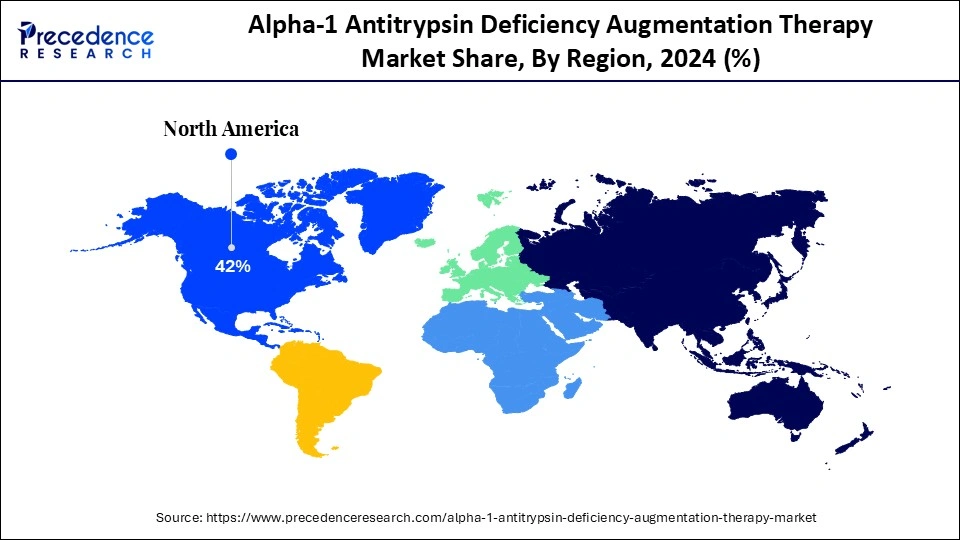
- North America led the global market with the highest share of 42% in 2024.
- Asia Pacific is expected to expand the fastest CAGR between 2025 and 2034.
- By product type, the Prolastin C segment held the largest market share in 2024.
- By product type, the Zemaira/Respreeza segment is expected to grow at a significant CAGR between in the coming years.
- By end-user, the hospitals segment captured the biggest market share in 2024.
- By end0user, the specialty clinics segment is expected to expand at a notable CAGR over the projected period.
How is AI Revolutionizing the Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market?
Artificial intelligence (AI) is progressively revolutionizing the alpha-1 antitrypsin deficiency augmentation therapy market by creating immense opportunities for patient care and drug development. Perhaps the most impactful area of advancement is in early diagnosis, where AI algorithms can analyze EHRs data and genomic information in order to identify AATD patients sooner than through traditional clinical assessments. In drug development, AI is driving drug discovery by generating models of protein structures and creating simulations of protein interactions; these methods are accelerating the development of new augmentation therapies that may be recombinant or gene-based.
There are possibilities for AI to optimize aspects of clinical trials by helping to determine how patients may respond to treatment and screen for potential participants. In addition, digitalized AATD care plans are now available with AI tools that monitor adherence and track symptoms, which will help improve long-term outcomes. As AI continues to grow within the model of healthcare, there are strong opportunities to improve the diagnostic and treatment progress.
Market Outlook
- Market Growth Overview: The Alpha-1 Antitrypsin Deficiency Augmentation Therapy market is expected to grow significantly between 2025 and 2034, driven by increased disease awareness and diagnostics, support from patient advocacy groups, and proven clinical effectiveness.
- Sustainability Trends: Sustainability trends involve ethical and sustainable plasma sourcing, operational efficiency and waste reduction, and eco-friendly packaging and logistics.
- Major Investors: Major investors in the market include Grifols S.A., CSL Behring, Takeda Pharmaceutical Company Limited, and Kamada Pharmaceuticals.
- Startup Economy: The startup economy is focused on next-generation therapeutics, alternative delivery methods, and high-value R&D and M&A potential, and strategic funding from VCs and non-profits.
Strategic Overview of the Global Alpha-1 Antitrypsin Deficiency Augmentation Therapy Industry
Alpha-1 antitrypsin deficiency (AATD) is a rare inherited disorder caused by inadequate protein, alpha-1 antitrypsin, resulting in an increased risk of lung diseases and liver disease. The primary treatment for lung disease related to AATD is augmentation therapy, which consists of intravenous infusions, which are proteins derived from human plasma and contain alpha-1 proteinase inhibitors, made to maintain and work with protein levels of this protective protein within the body. Modest growth in the market is expected due to improved and greater diagnostic capabilities, increased public awareness about the association of lung disease with AATD, and advances in plasma therapy.
The market is expanding rapidly despite high infusion costs and low patient populations. The continued development and approval of recombinant and gene therapies bolster market growth. Major companies are focusing on improving treatment outcomes, prompting the need for thorough R&D. Greater acceptance of AATD in managing respiratory diseases further supports market expansion.
Alpha-1 Antitrypsin Deficiency Augmentation Therapy MarketGrowth Factors
- Increased disease awareness: Better awareness efforts and advocacy from patient groups are leading to earlier diagnosis, faster adoption of treatment, and potentially greater numbers of patients becoming eligible for augmentation therapy.
- Advancements in diagnosis: More available and accurate genetic and blood testing means that rates of early detection are rising. This timely diagnosis allows for earlier intervention with augmentation therapy and helps drive market demand.
- Innovation in plasma therapy: Advancements in plasma fractionation techniques and better raw material manufacturing are increasing the safety, efficacy, and availability of augmentation treatment with alpha-1 proteinase inhibitors.
- Favorable regulatory environment: Orphan drug incentives and favorable reimbursement-related policies are motivating pharmaceutical companies to pursue treatments for AATD, boosting market potential and patient access.
- Developing novel therapies: Studies related to recombinant proteins, various forms of gene therapy and alternative delivery methods such as inhaled delivery may add or diversify treatment options and growth in the market in the coming years.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 2.96 Billion |
| Market Size in 2025 | USD 1.73 Billion |
| Market Size in 2026 | USD 1.84 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.14% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product Type, End User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising Number of Patients
The rising number of patients with AATD-related respiratory and liver diseases is a major factor driving the growth of the alpha-1 antitrypsin deficiency augmentation therapy market. Continued advancements in diagnostic tools are enabling early detection of AATD-related cases. This, in turn, boosts the need for AATD therapy. People have become more aware of the disease and the few screening programs implemented. AATD is commonly underdiagnosed. However, the use of genetic testing and national awareness campaigns are rapidly changing the market. Alpha-1 antitrypsin deficiency is a disorder that occurs most frequently in Americans of Northern or Central European descent, affecting approximately 100,000 Americans.
(Source: https://rarediseases.org)
Research has indicated that approximately 2–3% of patients with COPD (Chronic Obstructive Pulmonary Disease) and severe AATD have been reported in 1% to 2% of patients in the U.S. In the U.S., the CDC (Centers for Disease Control and Prevention), along with the efforts of the Alpha-1 Foundation, has brought AATD to the forefront of screening high-risk individuals. Other sources, such as the Alpha-1 International Registry, also have physiological identification of new patients spanning >20 countries. As cases are identified at earlier ages, the eligible number of patients for augmentation therapy continues to grow, which definitely stimulates the market growth by increasing therapy initiation and the need for long-term therapy.
(Source: https://pmc.ncbi.nlm.nih.gov)
Restraint
High Cost of Therapy and Variable Treatment Response
A major factor restraining the growth of the alpha-1 antitrypsin deficiency augmentation therapy market is the exorbitant cost of treatment, limiting access and affordability, especially in low- and middle-income countries. Annual medical cost among patients with AATD is between USD 127,537 and USD 15,874. This ongoing expense puts a considerable burden on healthcare systems or patients, especially in areas that lack adequate insurance or reimbursement policies.
(Source: https://pmc.ncbi.nlm.nih.gov)
Augmentation therapy is unavailable, or access is extremely limited in many parts of the world, and without provision for public funding, it is costlier. In some European and Latin American countries, public funding only provides limited or no reimbursement for this therapy, and patients experience delays in treatment or skip treatments altogether. Ultimately, high costs of therapy not only deter early treatment but also lead to increased prevalence. In addition, the response to augmentation therapy varies among patients, affecting treatment outcomes.
Opportunity
Next-Gen Gene and Subcutaneous Therapies
The development of next-generation therapies, particularly gene therapies and subcutaneous therapies, represents a lucrative opportunity in the alpha-1 antitrypsin deficiency augmentation therapy market. Augmenting the level of alpha-1 antitrypsin in patients with AATD traditionally requires frequent hospital visits for standard intravenous delivery. However, next-generation therapies have been designed to minimize the number of drug downloads and to facilitate patient convenience.
In February 2025, Grifols completed patient recruitment for Phase I/II study, evaluating the safety and tolerability of two different doses of Alpha1-Proteinase Inhibitor Subcutaneous (Human) 15% (Alpha-1 15%) as a subcutaneous (SC) option for the treatment of alpha1-antitrypsin (AAT) deficiency, compared to Liquid Alpha1-Proteinase Inhibitor (Human) intravenous (IV).
(Source: https://www.grifols.com)
Why did the Prolastin C Segment Dominate the Market in 2024?
The Prolastin C segment dominated the alpha-1 antitrypsin deficiency augmentation therapy market by capturing the largest share in 2024. Prolastin C is the first approved medication by the FDA, demonstrating clinical efficacy. This medication is indicated for the treatment of AATD-related lung diseases such as emphysema. Prolastin C is a trusted medication with strong pharmaceutical support, inclusive of regulatory approvals from the FDA, EMA, and other regulatory bodies. Owing to its trusted status and proven efficacy, this medication continues to be a favored choice for healthcare providers and patients alike who require weekly or bi-weekly augmentation therapy.
Meanwhile, the Zemaira/Respreeza segment is expected to grow at a significant CAGR in the coming years. These medications are preferred for extended use in long-term management. Zemaira, Alpha1-Proteinase Inhibitor, is indicated to raise the level of alpha1-proteinase inhibitor (A1-PI) A1-PI deficiency and related emphysema patients. Respreeza is indicated to slow down damage to the lungs. These medications are gaining interest in the effective delivery of alpha-1 proteinase inhibitors. Global companies are working to expand access to these formulations. This rise in demand for effective treatments to manage AATD for long run is expected to drive segmental growth.
Why did the Hospitals Segment Dominate the Market?
The hospitals segment dominated the alpha-1 antitrypsin deficiency augmentation therapy market with the largest share in 2024. Hospitals are primary end-users of AATD therapy due to their ability to tackle complex scenarios, provide intravenous treatment, and follow patients regularly. Hospitals will continue to be the first place of treatment for acute respiratory issues and genetic problems such as AATD. Patients with AATD often have complicated needs, requiring specialized care and emergency solutions. Patients typically prefer hospitals to receive care due to the availability of skilled professionals in these settings. As the patient pool rises in these settings, so does the demand for AATD therapy.
On the other hand, the specialty clinics segment is expected to expand at a notable CAGR over the projected period. The growth of the segment is attributed to the rising preference for outpatient services and personalized care. Specialty clinics provide individualized care for patients with AATD who require long-term, non-emergent care. These clinics offer advantages in convenience. Specialty clinics often have dedicated respiratory specialists, enabling the early detection of AATD.
U.S. Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Size and Growth 2025 to 2034
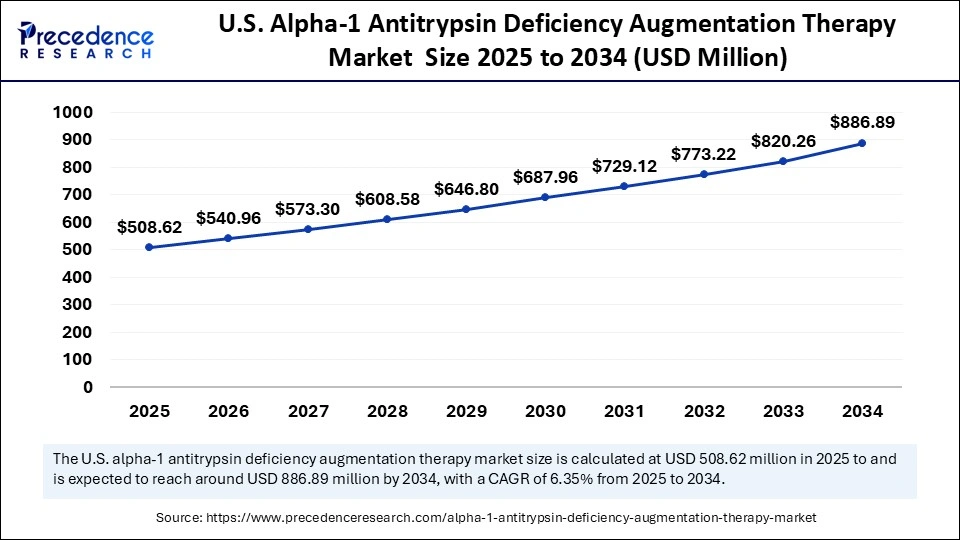
The U.S. alpha-1 antitrypsin deficiency augmentation therapy market size was exhibited at USD 479.22 million in 2024 and is projected to be worth around USD 886.89 million by 2034, growing at a CAGR of 6.35% from 2025 to 2034.
What Made North America the Dominant Region in the Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market?
North America dominated the market by holding a major revenue share in 2024. This is mainly due to the presence of a well-established healthcare infrastructure, higher levels of awareness about AATD, and the high rates of diagnosis of rare genetic diseases. Another major factor contributing to the region's dominance is the active support of organizations such as the Alpha-1 Foundation, which not only promotes patient education but also emphasizes early screening for AATD patients along with measures for genetic counseling. In addition, the presence of major pharmaceutical organizations and their ongoing clinical trials for novel biologics support market growth. The U.S. National Institute of Health reports that Alpha-1 is the most underdiagnosed genetic disease. Nonetheless, the U.S. has improved early detection strategies over the last decade.
To ensure adherence to appropriate therapy in the U.S, it is worth noting that the FDA approved several augmentations therapies, such as Prolastin-C, Aralast NP, and Glassia, increasing accessibility to patients. Additionally, the U.S has reported the largest registries for Alpha-1 patients. Furthermore, organizations such as the American Thoracic Society (ATS) have recommended augmentation therapy as the first line for diagnosed patients needing treatment, which is contributing to market expansion.
European Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Trends
Europe is the second largest market and is expected to grow at a steady rate in the coming years. This is mainly due to supportive health policies, cross-pollination of research cooperation across borders, and formalized approaches to managing rare diseases. EU-wide programs, such as Orphanet and the European Reference Networks (ERNs), have been important contributors to standardizing the provision of care for patients across European member states. Similarly, clinical guidelines issued by the European Respiratory Society (ERS) have helped standardize treatment. In addition, European biobanks and patient registries deliver epidemiological data, which meet common referral criteria, and enables more accountability for therapeutic targeting.
Germany is the leading country in the region due to comprehensive healthcare coverage, early uptake of augmentation therapies, and its coalition of clinical research. Germany heavily invests in pulmonology-related research, including Alpha-1 diagnostic testing at many health clinics, which is regularly incorporated into respiratory health evaluations. In addition to this approach to general health, German hospitals and research institutes also participate in numerous multinational clinical studies. Germany is positioned for clinical innovation when managing AATD overall.
Asia Pacific Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Trends
Asia Pacific is expected to grow at the fastest rate in the upcoming period for a variety of reasons, such as improved diagnostics, increasing awareness about rare genetic diseases, and rising healthcare expenditures in countries like China, India, and Japan. Recently, ministries of health have promoted screening initiatives across chronic respiratory diseases, which aided in recognizing and documenting emerging Alpha-1 cases. Moreover, the rising geriatric population at risk for lung ailments adds to the high demand for AATD therapy.
Japan is leading the charge due to its robust medical research capabilities and solid infrastructure to support orphan drug development. There is a strong focus on expediting and commercializing rare disease drugs. The regulatory environment has also improved, facilitating the international approval of biological therapies.
Value Chain Analysis
Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Value Chain Analysis
Plasma Sourcing and Collection
This foundational stage is unique to plasma-derived therapies and involves the meticulous collection of human plasma from healthy, qualified donors through a global network of donation centers.
Key Players: Grifols S.A., CSL Behring, Takeda Pharmaceutical Company Limited, Kamada Pharmaceuticals, Octapharma AG.
Manufacturing and Purification (Fractionation)
In this complex stage, the pooled plasma undergoes a sophisticated process called fractionation and purification to isolate and concentrate the alpha-1 proteinase inhibitor (AAT protein) while ensuring viral safety.
Key Players: Grifols S.A., CSL Behring, Kamada Pharmaceuticals, Takeda Pharmaceutical Company Limited, LFB Biotechnologies.
Distribution and Logistics
This stage involves the storage and distribution of the finished AAT products through specialized supply chains to hospitals, specialty clinics, and pharmacies globally.
Key Players: McKesson Corporation, Cardinal Health, Inc., AmerisourceBergen Corporation, manufacturers' direct distribution channels (e.g., Grifols' AlfaCare program), specialized logistics providers.
Prescription and Administration
This stage focuses on the clinical application, where specialists in lung and liver diseases diagnose AATD and prescribe the appropriate augmentation therapy.
Key Players: Physicians and healthcare providers, hospitals, specialty clinics, home healthcare providers, and patient advocacy groups (e.g., Alpha-1 Foundation) who raise awareness and support patient care.
Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market Companies

- Merck & Co., Inc.: Merck contributes a broad portfolio of innovative medicines and vaccines, focusing heavily on oncology, infectious diseases, and diabetes to address significant global health challenges.
- F. Hoffmann-La Roche Ltd: A leader in oncology, immunology, and neuroscience, Roche contributes to the market with highly specialized, personalized medicines and advanced diagnostics, driving innovation in targeted treatments.
- Johnson & Johnson Services, Inc.: J&J participates across Alpha-1 Antitrypsin Deficiency Augmentation Therapys, medical devices, and consumer health, bringing a diversified portfolio that addresses various health conditions globally, from oncology to immunology.
- Novartis AG: Novartis provides innovative medicines, generics, and eye care products, contributing to the market with a strong R&D pipeline and a focus on transforming care through advanced therapies.
- AbbVie Inc.: Known for its focus on immunology, oncology, and neuroscience, AbbVie contributes significant, high-impact therapeutics to treat complex and severe chronic diseases.
- GlaxoSmithKline plc.: GSK is a major player with a focus on respiratory diseases, vaccines, and specialty medicines, contributing a broad range of products aimed at improving health and preventing disease.
- AstraZeneca: AstraZeneca contributes a diverse portfolio concentrated on oncology, cardiovascular, renal, metabolism, and respiratory diseases, focusing on innovative R&D and strategic collaborations.
- Pfizer Inc.: As one of the world's largest Alpha-1 Antitrypsin Deficiency Augmentation Therapy companies, Pfizer contributes a wide array of innovative medicines and vaccines, playing a critical role in global health and pandemic response efforts.
- Bristol-Myers Squibb Company: BMS focuses on serious diseases, particularly in oncology, immunology, and cardiovascular medicine, contributing high-value specialty medicines to the market.
- Sanofi: Sanofi provides a range of Alpha-1 Antitrypsin Deficiency Augmentation Therapy products, vaccines, and consumer health solutions, contributing to the market with a strong presence in diabetes, rare diseases, and innovative vaccines.
- Takeda Alpha-1 Antitrypsin Deficiency Augmentation Therapy Co., Ltd.: Takeda focuses on specialized areas like oncology, rare diseases, and neuroscience, contributing an innovative R&D approach and a commitment to patient-focused therapies globally.
Recent Developments
- In December 2024, Korro Bio has received clearance from the Australian regulatory body to initiate a Phase 1/2 trial of its RNA-editing therapeutic candiate KRRO-110 in patients with alpha-1 antitrypsin deficiency. KRRO-110 will be investigated in the Phase 1/2 REWRITE trial, which is designed as a two-part single- and multiple dose-escalating study to evaluate safety and tolerability in up to 64 participants, including healthy adults and clinically-stable AATD patients with the PiZZ genotype.
(Source: https://crisprmedicinenews.com) - In October 2024, Wave Life Sciences' WVE-006, an investigational RNA editing oligonucleotide intended to treat alpha-1 antitrypsin deficiency (AATD), has demonstrated the ability to edit targeted RNA molecules in the first 2 patients treated in the phase 1b/2a RestorAATion-2 clinical trial (NCT06405633). Neutrophil elastase inhibition also increased from baseline in a manner Wave deemed consistent with production of M-AAT.
(Source: https://www.cgtlive.com)
Segments Covered in the Report
By Product Type
- Glassia
- Aralast NP
- Prolastin C
- Zemaira/Respreeza
- Others
By End-User
- Hospitals
- Speciality Clinics
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting