Cloud-Based Clinical Trial Market Size and Forecast 2025 to 2034
The cloud-based clinical trial market is witnessing strong growth as sponsors and CROs adopt cloud technologies to enable real-time collaboration, streamline data management, and support decentralized and hybrid trial models. The growth of the market is attributed to the rising adoption of decentralized clinical trials.
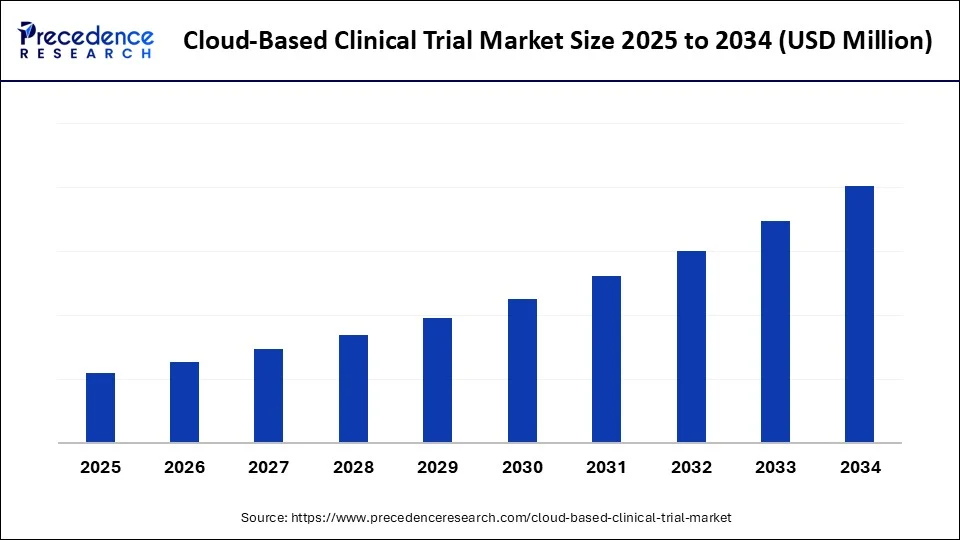
Cloud-Based Clinical Trial Market Key Takeaways
- North America dominated the cloud-based clinical trial market, holding a 49.50% share in 2024.
- Asia Pacific is expected to expand at the fastest CAGR in the market between 2025 and 2034.
- By component, the software segment led the market while holding 29.50% share in 2024
- By component, the e-consent platforms segment is expected to grow at a significant CAGR between 2025 and 2034.
- By deployment model, the private cloud segment led the market while holding a 47.50% share in 2024
- By deployment model, the hybrid cloud segment is expected to grow at a remarkable CAGR between 2025 and 2034.
- By application, the phase III trials segment led the market while holding 37.50% share in 2024
- By application, the phase I trials segment is expected to grow at the fastest CAGR between 2025 and 2034.
- By trial design, the traditional trial segment led the market while holding a 53.50% share in 2024.
- By trial design, the virtual/DCTs segment is expected to grow at the fastest CAGR between 2025 and 2034.
- By end-user, the pharma & biotech companies segment led the market while holding a 42.50% share in 2024.
- By end-user, the CROs segment is expected to grow at a significant CAGR between 2025 and 2034.
- By delivery model, the SaaS segment led the market while holding a 63.50% share in 2024.
- By delivery model, the PaaS segment is expected to grow at a remarkable CAGR between 2025 and 2034.
Impact of AI on the Clod-Based Clinical Trial Market
Artificial intelligence is transforming the cloud-based clinical trial market with unprecedented speed and precision by automating patient recruitment, predicting outcomes, and analyzing vast datasets in real time. AI significantly reduces both time and costs. Machine learning models are helping researchers identify patterns and insights that were previously undetectable. AI-driven virtual assistants and chatbots are enhancing patient engagement and compliance throughout the trial. As a result, trials are becoming faster, more efficient, and increasingly personalized.
Market Overview
The cloud-based clinical trial market encompasses the ecosystem of technologies, platforms, and services that utilize cloud computing to support various phases and operations of clinical trials, including trial design, site selection, data capture, remote monitoring, regulatory compliance, and analytics. These platforms improve trial efficiency, enable real-time access to data, enhance collaboration across stakeholders, support decentralized and virtual trials, and ensure scalability, security, and compliance.
The cloud-based clinical trial market is revolutionizing how medical research is conducted by offering scalable, real-time digital solutions for data collection, management, and collaboration. The market is experiencing robust growth, driven by the increasing need for remote trials and real-time data access. The rising adoption of decentralized trials post-pandemic has further accelerated this shift. Cloud platforms allow seamless integration of EHRs, wearable device data, and patient-reported outcomes, offering a holistic trial ecosystem. Regulatory bodies are also becoming increasingly accepting of cloud technologies, which support smoother compliance processes. As sponsors strive to reduce costs and optimize clinical trials, cloud solutions are becoming increasingly indispensable.
Key Market Trends
- Shift towards decentralized trials: Sponsors are increasingly adopting decentralized clinical trial models enabled by cloud platforms that support remote patient monitoring, virtual visits, and digital consent.
- Integration of wearables and IoT devices: Wearable health tech and IoT devices are now being integrated into trial platforms, streaming continuous patient data into the cloud for real-time analysis and reporting.
- Emphasis on data security and compliance: With vast patient data being stored and transferred online, there's a growing focus on enhancing cybersecurity measures and maintaining compliance with regulations like GDPR and HIPAA.
- AI and machine learning adoption: Cloud-based trials are increasingly using AI/ML tools for protocol optimization, patient matching, and outcome prediction, reducing manual effort and improving trial efficiency.
- Growth of hybrid trial models: Sponsors are combining traditional and virtual methods to create hybrid trial designs, offering the flexibility of cloud-based systems while retaining some on-site touchpoints.
- Global collaboration and scalability: Cloud infrastructure is making it easier for trial sponsors to run global, multicenter trials by providing scalable, unified platforms for data sharing and coordination.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Component, Deployment Model, Application, Trial Design, End Use, Delivery Model, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increased Need to Reduce Trial Costs and Enhance Collaboration
One of the key factors driving the growth of the cloud-based clinical trial market is the increasing demand for faster and more cost-effective trials. Traditional clinical trial models are expensive, rigid, and often delayed, whereas cloud platforms offer flexibility, automation, and global accessibility. Pharmaceutical and biotech companies are under increasing pressure to accelerate drug development, especially in the face of emerging diseases. Cloud-based systems streamline data collection, enable real-time analytics, and reduce manual errors, significantly cutting down trial duration. Moreover, the rise of personalized medicine and complex trial designs demands platforms that can integrate and manage massive, varied datasets. Cloud-based solutions facilitate collaboration among different stakeholders, such as sponsors, researchers, and CROs, accelerating clinical trials and drug development.
Restraint
Data Privacy Concerns and Regulatory Compliance
Despite several benefits of cloud-based clinical trials, the market faces several challenges that hinder growth. Data privacy and security concerns are one of the major challenges. Clinical trials often deal with sensitive patient information, which is stored and shared across borders. This raises concerns about data security. Many clinical researchers and institutions still show resistance to adopting new cloud technologies due to a lack of training or fear of losing control over data. Additionally, varying global regulations create compliance hurdles for companies running multinational cloud trials. Interoperability issues between legacy systems and modern cloud platforms can also disrupt workflow and delay trial timelines. Moreover, high upfront investment and unclear return on investment in the early stages may deter small sponsors.
Opportunity
Expansion into Emerging Markets
There is a significant opportunity to expand decentralized clinical trial solutions into emerging markets with limited clinical infrastructure. Cloud platforms enable remote trials, providing access to diverse patient populations across the Asia Pacific, Africa, and Latin America. In addition, the rise of digital therapeutics and mobile health technologies creates new categories of trials that cloud systems are uniquely suited to support. Companies are offering specialized AI-driven cloud platforms for protocol simulation and adaptive trial management to gain a competitive advantage. Furthermore, as regulatory bodies become more accepting of virtual trials, a new wave of regulatory tech integration is poised to grow. This evolution is paving the way for more inclusive, agile, and technology-driven research ecosystems.
Component Insights
Why Did the Software Segment Dominated the Market in 2024?
The software segment dominated the cloud-based clinical trial market with the largest share in 2024, under which electronic data capture (EDC) remains the leading sub-segment. This is mainly due to the rapid shift toward decentralized clinical trials. EDC software is in high demand due to its ability to simplify and centralize data collection between sites, sponsors, and regulators. It also improves data accuracy and reduces trial timelines significantly. The user-friendly interfaces and real-time analytics make EDC software the preferred choice for sponsors. As trial complexity increases, reliance on advanced software solutions like EDC will grow.
On the other hand, the e-consent platforms segment is expected to grow at the fastest rate in the upcoming period. These platforms offer an all-in-one suit combining EDC, CTMS, ePRO, and more. Sponsors are shifting from soiled tools to integrated ecosystems that offer real-time insights and improved trial oversight. The ability to centralize workflows and automate routine tasks is a major advantage. These platforms also improve protocol adherence and reduce human error. With scalability and flexibility, e-clinical platforms are quickly becoming the industry standard.
Deployment Model Insights
How Does the Private Cloud Segment Dominate the Cloud-Based Clinical Trial Market in 2024?
The private cloud segment dominated the market with a major revenue share in 2024. This is mainly due to the increased concerns over data security. Private cloud deployment offers superior data control, making it ideal for handling sensitive clinical data. Pharma companies and CROs favor private setups for security, compliance, and customization. Unlike the public cloud, private setups allow more tailored data governance strategies. They also ensure better alignment with strict regulatory requirements. This makes the private cloud the dominant deployment model across large-scale clinical operations.
Meanwhile, the hybrid cloud segment is expected to grow at the fastest CAGR during the projection period. The growth of the segment is attributed to the rising need for enhanced flexibility and collaboration as well as concerns over data privacy. A hybrid cloud setup combines the security of a private cloud with the scalability of public infrastructure. This setup supports dynamic workloads, remote access, and cost flexibility. Sponsors prefer hybrid models when running global or multi-phase studies. The model allows for secure core data storage while enabling public access for analysis and collaboration. As trial complexity increases, hybrid cloud adoption is accelerating rapidly.
Application Insights
What Made Phase III Trials the Dominant Segment in the Market in 2024?
The phase III trials segment dominated the cloud-based clinical trial market, capturing the largest share in 2024. This is mainly due to their large scale and regulatory importance. These trials generate the most data, making cloud platforms essential for efficient management. Sponsors rely on cloud systems for centralized control, real-time data review, and faster decision-making.
On the other hand, the phase I trials segment is expected to grow at the fastest CAGR in the coming years. Early use of cloud systems helps streamline safety assessments and dose escalation decisions. Real-time monitoring improves trial safety and responsiveness. Sponsors are using ePRO and remote monitoring even at this early stage. Cloud platforms ensure transparency and efficiency right from trial initiation. This digital-first approach is shaping the future of early-phase research.
Trial Design Insights
Why Did the Traditional Trials Segment Dominate the Cloud-Based Clinical Trial Market in 2024?
The traditional trials segment dominated the market in 2024 primarily due to their established methodologies and regulatory acceptance. They have a long history of effectiveness in demonstrating safety and efficacy, which fosters trust among stakeholders, including regulators, healthcare professionals, and patients. The extensive data generated from these trials often leads to robust and reliable outcomes, making them the gold standard in clinical research. Additionally, traditional trials benefit from significant funding and resources, which further solidify their position in the industry. The familiarity of stakeholders with the structure and processes involved in traditional trials also contributes to their continued preference. Lastly, the rigorous oversight and adherence to ethical standards ensure that participant safety and data integrity remain paramount, adding to their credibility in the market.
On the other hand, the virtual/decentralized clinical trials (DCTs) segment is likely to grow at the fastest rate in the upcoming period. Cloud-based platforms make it possible to recruit, monitor, and engage patients without requiring on-site visits. This model improves accessibility, especially for rare diseases and rural populations. It also reduces participation dropout by offering more convenience. Sponsors are investing in remote tools like e-consent, telehealth, and wearable integrations. Decentralized clinical trials (DCTs) are not just a trend; they are refining how trials are designed and delivered.
End-User Insights
How Does the Pharma & Biotech Companies Segment Dominate the Market in 2024?
The pharma & biotech companies segment dominated the market with a major share in 2024. They require high data security, scalability, and compliance, all of which cloud systems provide. With growing pipelines and pressure to accelerate development, cloud-based trials offer unmatched agility. These companies are investing heavily in AI-powered cloud solutions for protocol design and data interpretation. Cloud systems also support collaboration with global trial partners. As innovation grows, pharma and biotech will continue to shape the market.
Meanwhile, the contract research organizations (CROs) segment is expected to expand at a rapid pace during the projection period. CROs are rapidly adopting cloud platforms to enhance service delivery. Cloud tools allow them to manage multiple clients' trials with capitalized oversight. They enable faster reporting, clearance data, and better compliance tracking. By offering cloud-enabled solutions, CROs increase their competitiveness and value to sponsors. Automation and integration across sites also reduce operational costs. This makes CROs a major force in scaling the cloud trial ecosystem.
Delivery Model Insights
Why Did the Software-as-a-Service (SaaS) Segment Dominate the Market?
The software-as-a-service (SaaS) segment dominated the cloud-based clinical trial market in 2024 due to its ability to streamline clinical trial processes. It allows quick deployment, easy access, and reduced IT burden for sponsors and sites. SaaS platforms are flexible and often come with automatic updates and support. They are ideal for sponsors seeking fast, scalable solutions across trials. With rising trial volumes, SaaS is becoming the go-to model for speed and simplicity. Its subscription-based pricing also fits well with trial budgets.
On the other hand, the platform-as-a-service (PaaS) segment is expected to grow at the fastest CAGR in the upcoming period due to its ability to support customized trial tools. Sponsors use PaaS to build, test, and deploy applications tailored to their study needs. It offers more control than SaaS, especially for complex or high-security trials. PaaS also facilitates the integration of AI, wearables, and analytics into trial systems. Though adoption is smaller than SaaS, it's growing among large enterprises. PaaS is ideal for sponsors who want flexibility and long-term control.
Regional Insights
What Made North America the Dominant Region in the Market?
North America dominated the cloud-based clinical trial market by capturing the largest share in 2024. This is mainly due to the increased volume of clinical trials. With robust healthcare clinical infrastructure, there is a high adoption of cloud-based solutions to decentralize clinical trials. The region is home to a large number of pharma companies and CROs, which are actively taking part in clinical trials. The region benefits from robust regulatory support and high digital literacy. Remote patient monitoring and high-tech tools are rapidly being integrated into trial workflows. The U.S., especially, is pushing decentralized trials and cloud-based data sharing. The rising drug development further supports the region's dominance.
What are the Major Factors Driving the Growth of the Cloud-Based Clinical Trial Market in Asia Pacific?
Asia Pacific is emerging as the fastest-growing region in the market due to the rising clinical trial activities. Due to the vast, diverse population and cost-effective trial setups, global sponsors are shifting to this region to conduct clinical trials. Governments around the region are investing heavily in clinical research, boosting the adoption of cloud-based solutions. There is a high adoption of mobile health, helping cloud-based platforms gain traction. Regulatory reforms are supporting decentralized and hybrid models. Furthermore, increasing collaborations between local and international research organizations to conduct clinical trials supports regional market growth.
Cloud-Based Clinical Trial Market Companies

- Medidata Solutions (Dassault Systèmes)
- Veeva Systems
- Oracle Health Sciences
- Parexel International
- ICON plc
- IQVIA
- eClinicalWorks
- Signant Health
- CRF Health (merged with Bracket to form Signant)
- Medrio
- IBM Watson Health
- Clario
- Castor EDC
- OpenClinica
- ArisGlobal
- Bio-Optronics
- Clinical Ink
- Advarra
- Cloudbyz
- RealTime Software Solutions
Recent Development
- In January 2025, EDETEK announced the launch of R&D Cloud to improve the clinical research process. Built upon the company's core platforms, CONFORM™ and eClinical, R&D Cloud is a versatile solution that users can alter to fit a specific study's needs from start to finish.
(Source: https://www.pharmexec.com)
Segments covered in the report
By Component
- Software
- Clinical Trial Management Systems (CTMS)
- Electronic Data Capture (EDC)
- Electronic Patient-Reported Outcomes (ePRO)
- Randomization and Trial Supply Management (RTSM)
- eConsent Platforms
- Data Analytics & Visualization Tools
- Services
- Deployment and Integration Services
- Training and Support
- Managed Services
- Cloud Hosting Services
By Deployment Model
- Public Cloud
- Private Cloud
- Hybrid Cloud
By Application
- Phase I Trials
- Phase II Trials
- Phase III Trials
Phase IV/Post-Marketing Surveillance
By Trial Design
- Traditional Clinical Trials
- Virtual/Decentralized Clinical Trials (DCTs)
- Hybrid Clinical Trials
By End Use
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic & Research Institutes
- Medical Device Companies
By Delivery Model
- Software-as-a-Service (SaaS)
- Platform-as-a-Service (PaaS)
- Infrastructure-as-a-Service (IaaS)
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content
 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting