What is the Duchenne Muscular Dystrophy Drugs Market Size?
The global duchenne muscular dystrophy drugs market size is accounted at USD 4.79 billion in 2025 and is predicted to increase from USD 5.60 billion in 2026 to approximately USD 19.46 billion by 2034, expanding at a CAGR of 16.85% from 2025 to 2034. The Duchenne muscular dystrophy drugs market is growing due to advancements in genetic therapies, rising global demand for effective treatments, and a strong pipeline of emerging drugs.
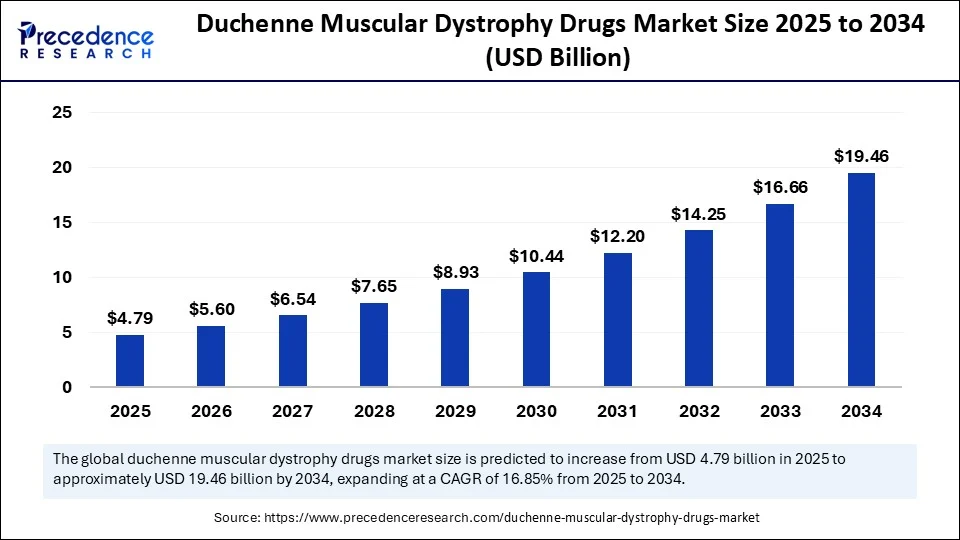
Duchenne Muscular Dystrophy Drugs MarketKey Takeaways
- North America dominated the duchenne muscular dystrophy drugs market with the largest market share of 46% in 2024.
- Asia Pacific is expected to witness the fastest growth during the forecast years.
- By treatment, the molecular based therapies segment held the biggest market share of 43% in 2024.
- By treatment, the steroid based therapies segment is anticipated to show a considerable CAGR of 16.25% over the forecast period.
- By distribution channel, the hospital pharmacies segment held a significant market share of 42% in 2024.
- By distribution channel, the online pharmacies segment is expected to grow at a solid CAGR of 16.74% during the forecast period.
Market Overview
Duchenne Muscular Dystrophy (DMD) is a severe, progressive genetic disorder characterized by muscle loss and reduced strength due to a deficiency of dystrophin, a critical protein for maintaining cell integrity. Primarily affecting young boys, symptoms typically emerge between ages 3 and 5, with initial signs including delayed motor development and difficulty walking or talking. While currently incurable, significant medical advancements have led to the development of various pharmaceutical interventions. These interventions focus on managing symptoms, slowing disease progression, and improving the quality of life.
The rapid advancements in genetic research and molecular biology are enabling the development of targeted treatments, such as exon-skipping medications and gene-editing drugs, which are yielding remarkable outcomes. Increased funding from governments, non-profit organizations, and pharmaceutical companies is accelerating innovation. Furthermore, regulatory bodies are adopting more flexible guidelines, which speed up the approval process for new treatments. Current DMD medications include corticosteroids, exon-skipping drugs, and emerging gene-based therapies.
How is AI Transforming the Duchenne Muscular Dystrophy Drugs Market?
The market is evolving with the application of artificial intelligence in drug discovery and development. AI supports the analysis of vast data sets more quickly and accurately to identify disease patterns. AI is also enhancing clinical trial design and patient recruitment, analyzing real-world data to find the right patient population and predict treatment success.Additionally, AI-based systems help process genetic information to create personalized treatment plans based on individual patient mutations and disease progression. The use of AI by pharmaceutical and biotech companies is increasing to improve R&D efficiency, decision-making, and the time to market for innovative drugs.
What Factors are Fueling the Growth of the Duchenne Muscular Dystrophy Drugs Market?
- Increasing Awareness of DMD: With increased awareness and improved diagnosis, more patients are being identified, which is driving the development of specialized therapies. This trend is encouraging pharmaceutical companies to focus on DMD drug development to address the urgent and unmet medical needs associated with this severe genetic disorder.
- Genetic and Molecular Research Development: The groundbreaking advancements in genetic engineering, exon skipping, and molecular biology have revolutionized the approach to DMD. Recent innovations like CRISPR gene editing and RNA-specific therapies have shown high specificity and hold potential for a cure.
- Favorable Regulatory Systems: Regulatory bodies are working to expedite procedures and provide special designations (including Orphan Drug and Fast Track status) for DMD treatments. These policies encourage innovation by reducing time to market and offering incentives such as extended market exclusivity.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 19.46 Billion |
| Market Size in 2026 | USD 5.60 Billion |
| Market Size in 2025 | USD 4.79 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 16.85% |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Treatment, Distribution Channel, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Increasing prevalence of DMD
The increasing prevalence of Duchenne muscular dystrophy (DMD) globally, along with the rise in newborn screening programs, increased disease awareness, and growth in research activities, are key drivers in the Duchenne muscular dystrophy drugs market. Early and accurate diagnosis is crucial for DMD treatment, as early intervention slows the disease's progression and improves quality of life. Advocacy groups and healthcare organizations have organized awareness campaigns that enhance understanding of DMD symptoms, encourage early medical visits, and reduce the time to diagnosis. Additionally, educational programs are increasing caregivers' and clinicians' understanding of the importance of early genetic testing.
- In April 2024, Ohio Governor Mike DeWine proclaimed that Ohio would be the first state in America who start screening all newborn children for Duchenne Muscular Dystrophy (DMD).
Gene Therapy Innovations
Genetic treatments are becoming one of the most revolutionary forces compared to traditional treatment methods, which focus on treating symptoms. Gene therapy targets the disease's root cause by introducing functional copies of the defective dystrophin gene. Since DMD is caused by mutations in the gene responsible for producing the dystrophin protein, which maintains muscle cell integrity, restoring this gene's expression can halt or significantly slow the disease's progression. A key development in DMD gene therapy involves adeno-associated virus (AAV) vectors, which are becoming a safer and more effective way to deliver therapeutic genes to muscle tissues.
Restraint
High Cost and Stringent Regulatory Approval
The high costs associated with the manufacturing of Duchenne Muscular Dystrophy (DMD) drugs are a major factor hampering the growth of the market. As there are no curable drugs, developing effective drugs requires thorough R&D and clinical trials. The high cost of treatments and the limited efficacy of existing therapies create challenges in the market. Moreover, stringent regulatory requirements and lengthy approval processes limit the accessibility, restraining the growth of the market.
Opportunities
No Cure for the Progressive Disorder
There is still no cure for Duchenne Muscular Dystrophy, creating immense opportunities in the Duchenne muscular dystrophy drugs market. The gradual and irreversible muscle degeneration leads to a progressive decline in muscle strength and function. Current treatments primarily address symptoms or offer limited slowing of the condition's progression without preventing or reversing the disease itself. This lack of curative treatments creates opportunities for market players to develop innovative treatments.
Emerging Combination Therapies
The pathology of DMD involves not only muscular degeneration but also extra-muscular complications like inflammation, fibrosis, and respiratory or cardiac issues. Single-agent therapies have not been very effective in addressing the entire disease. Corticosteroids, like prednisone and deflazacort, slow muscle breakdown and reduce inflammation, while exon-skipping drugs aim to partially restore dystrophin synthesis. Combination therapies are being developed as scientists gain a better understanding of DMD's biology, recognizing that there is no single cure. Clinical trials are underway to assess the safety and efficacy of these multi-modal therapies, offering hope for improved long-term outcomes.
Segment Insights
Treatment Insights
Why Did the Molecular Based Therapies Segment Dominate the Duchenne Muscular Dystrophy Drugs Market in 2024?
The molecular based therapies segment dominated the Duchenne muscular dystrophy drugs market and accounted for the largest revenue share of 43% in 2024. This is mainly due to the increased number of patients with DMD, as well as the trend towards the use of solutions that focus on the genetic cause of the disease. Molecular therapeutics offer individualized, targeted treatments based on patients' specific genetic variations.
The dominance of this segment is reinforced by advancements in molecular biology and a strong pipeline of new treatments. Major pharmaceutical companies are investing heavily in novel technologies like antisense oligonucleotides and gene-based therapies to enhance the molecular treatment profile. Furthermore, the increasing awareness and clinical adoption of precision medicine are boosting the use of these therapies, ensuring the long-term growth of the segment.
The steroid based therapies segment is expected to grow at the fastest CAGR of 16.25% over the forecast period. Steroids, particularly corticosteroids like prednisone and deflazacort, are central to DMD treatment because they reduce muscle degeneration and improve patient mobility. These steroid-based products remain crucial due to their ease of use, low cost, and widespread clinical experience. Recent advancements in steroid development have made them less prone to side effects and increased patient tolerance, further enhancing their use. The combination of corticosteroids with new treatments like exon-skipping drugs and gene therapies is also increasing their effectiveness in combination regimens. Several combination strategies are in clinical development, potentially offering synergistic benefits.
Distribution Channel Insights
How Does the Hospital Pharmacies Segment Dominate the Market in 2024?
The hospital pharmacies segment dominated the Duchenne muscular dystrophy drugs market with the biggest revenue share of 42% in 2024. Hospital pharmacies play a crucial role in DMD treatment and management by ensuring the safe, timely, and efficient dispensing of highly specialized medications like corticosteroids, exon-skipping medicines, and novel gene therapies. The increased availability of new drugs and customized therapies for DMD has made hospital pharmacies more important as distribution centers. They offer a safe and effective supply chain, handling the storage and delivery of expensive biologics and gene therapies, making them a preferred delivery channel.
The online pharmacies segment is expected to grow at the fastest CAGR of 16.74% in the coming years. Online pharmacies are becoming an accessible and convenient option for DMD treatments, with a growing trend in online stores offering health products and services. This growth is fueled by the increasing use of e-commerce and digital health channels. Online pharmacies offer benefits like home delivery, 24-hour availability, a wider selection of medicines, and sometimes competitive pricing.
The rise of telemedicine and internet-prescription services further supports this trend. As more people access the internet and use smartphones, online pharmacies will capture a larger share of the DMD drug market, especially in areas with limited hospital access.
Regional Insights
U.S. Duchenne Muscular Dystrophy Drugs Market Size and Growth 2025 to 2034
The U.S. duchenne muscular dystrophy drugs market size is exhibited at USD 1.54 billion in 2025 and is projected to be worth around USD 6.38 billion by 2034, growing at a CAGR of 17.06% from 2025 to 2034.

What Made North America the Dominant Region in the Duchenne Muscular Dystrophy Drugs Market in 2024?
North America registered dominance in the market by capturing the largest share of 46% in 2024. This is mainly due to a strong healthcare infrastructure and research institutions supporting clinical trials and drug development programs. An attractive regulatory environment, with streamlined review pathways and orphan drug designation, encourages pharmaceutical companies to invest in DMD research and commercialization.
The U.S. is leading the market in North America due to its healthcare expenditure and infrastructure. The U.S. market benefits from significant investment in rare disease research, including DMD, enabling extensive clinical studies by major medical organizations. Additionally, well-developed patient advocacy groups raise awareness, improve diagnosis, and enhance treatment options.
In March 2024, Catalyst Pharmaceuticals, Inc. announced the U.S. commercial distribution of AGAMREE oral suspension, 40 mg/mL dd, that is used to treat DMD in patients 2 years and above. The AGAMREE is named, subject to FDA approval on October 26, 2023, and is being offered by prescription and distributed nationwide with a network of specialized pharmacies. The launch was a major step towards increasing the number of treatments available to DMD patients in the U.S., thereby enhancing access to essential treatments.(Source: https://www.parentprojectmd.org)

Why is Asia Pacific Experiencing the Fastest Growth?
Asia Pacific is expected to grow at the fastest CAGR during the forecast period. Increased awareness of rare diseases, improved diagnostic capabilities, and growing healthcare spending drive market growth. Countries like Japan, China, and India are leading regional development through increased investments in health facilities and research into new DMD treatments. Governments are also implementing supportive policies and funding programs to improve rare disease management, which is making the market favorable to expansion.
China is the largest and most effective market in the Asia Pacific region, driven by rapid healthcare system restructuring and increased focus on rare disease treatments. With government support, patient advocacy, and a focus on innovation, China is playing a significant role in the future of DMD drugs in the Asia Pacific and is at the forefront of regional growth.
What Are the Key Trends in the European Duchenne Muscular Dystrophy Drugs Market?
The European Duchenne muscular dystrophy drugs market is expected to grow at a notable rate in the coming years. Well-developed medical care systems and regulatory frameworks in the region are key factors in the transformation and creation of new DMD therapies and treatments. Government programs and special funding for rare disease research have accelerated clinical research and streamlined regulatory processes, facilitating faster market access for new medicines.
Germany leads the market due to its robust healthcare system and focus on rare disease management. The nation is deeply engaged in developing innovative treatments, with strong collaboration among government agencies, academic institutions, and pharmaceutical companies. Moreover, Germany's strong reimbursement policies and healthcare access programs ensure patients have early access to the latest DMD treatments.
Why Are the Middle East & Africa Gaining Momentum in Duchenne Muscular Dystrophy Drug Adoption?
As awareness for rare diseases is increasing and diagnostic abilities are improving, there is an increasing focus on Duchenne muscular dystrophy (DMD) treatment in the Middle East & Africa. Governments and private hospitals are launching expanded genetic-testing programs that make it easier to establish the diagnosis of DMD. Partnerships with research institutes and advocacy groups also help increase access to clinical trials and education regarding the treatment of DMD. An increase in funding of pediatric neuromuscular care centers and availability of supportive therapies is also contributing to demand.
The UAE stands out with strong programs on rare disease, good genomic screening, and partnerships with global biotechnology developers. Abu Dhabi and Dubai have child neuromuscular clinics that provide early diagnosis and structured care pathways for DMD. The UAE is also prioritizing modern therapeutics and expedited access to generative approvals.
Ongoing Clinical Trials Duchenne Muscular Dystrophy Drugs (2023-2024)
|
Study Title |
Study Status |
Sponsor |
Phases |
Start Date |
Completion Date |
|
Sodium/Glucose Cotransporter-2 Inhibitors (SGLT2i) Therapy in Duchenne Cardiomyopathy |
Not Yet Recruiting |
Larry W. Markham |
PHASE1 |
2/1/2026 |
2/1/2028 |
|
Pharmacokinetics and Safety of Givinostat in DMD Patients Ages From at Least 2 Years to Less Then 6 Years Old |
Recruiting |
Italfarmaco |
PHASE2 |
1/2/2025 |
12/1/2029 |
|
A Study of PGN-EDO51 or Placebo in People With Duchenne Muscular Dystrophy Amenable to Exon 51-Skipping Treatment |
Withdrawn |
PepGen Inc |
PHASE2 |
12/17/2024 |
5/28/2025 |
|
A Study on Safety and Effectiveness of Long-term Treatment With Vamorolone in Boys With Duchenne Muscular Dystrophy |
Recruiting |
Santhera Pharmaceuticals |
PHASE4 |
11/10/2024 |
9/1/2028 |
|
Vasodilator and Exercise Study for DMD (VASO-REx) |
Recruiting |
University of Florida |
PHASE2 |
6/5/2024 |
11/1/2026 |
Value Chain Analysis for Duchenne Muscular Dystrophy Drugs Market
Discovery and Early Research and Development: Early discovery involves building a target biology, oligonucleotides, AAV constructs, and biomarkers to develop candidate therapeutics (exon-skipping therapies, read-through agents, gene replacement).
- Key Players:Sarepta Therapeutics, PTC Therapeutics, Solid Biosciences, Capricor Therapeutics, and Wave Life Sciences.
Preclinical and Clinical Development: Preclinical models and staged clinical trials in humans evaluate safety, dosing, and efficacy; all are informed with the help of natural history studies and patient registries.
- Key Players: Parexel, ICON, IQVIA, PRA Health Sciences, Covance.
Biomanufacturing and Formulation: Scale-up; AAV/viral vector production; cell therapy manufacturing and analytics; all services provide consistency in GMP drug product supply for sponsors to conduct trials and seek commercialization.
- Key Players: Catalent, Lonza, Thermo Fisher Scientific, Samsung Biologics, Fujifilm Diosynth.
Regulatory Approvals, Licensing and Partnerships: Regulatory approvals, safety monitoring, advisory panels, licensing agreements, indicate market access; and post-approval label updates and risk-management plans.
- Key Players: Sarepta, PTC Therapeutics, Capricor, major regulators (FDA/EMA ) and regulatory consultancies.
Distribution and Patient Support: Specialty pharmacy/restorative pharmacy; distribution; and patient-support programs provide cold-chain delivery, reimbursement navigation, home infusion coordination, along with long-term follow-up.
- Key Players: CVS Specialty/Accredo, Walgreens Specialty, McKesson Specialty Health, Cardinal Health, and Patient advocacy groups (MDA, PPMD).
Duchenne Muscular Dystrophy Drugs Market Companies

- Aurobindo Pharma
- Capricor Therapeutics, Inc.
- Catalyst Pharmaceuticals, Inc.
- EspeRare Foundation
- FibroGen, Inc.
- ITALFARMACO S.p.A.
- NS Pharma, INC.
- PTC Therapeutics.
- Santhera Pharmaceuticals
- Sarepta Therapeutics, Inc.
Recent Developments
- In March 2025, Capricor Therapeutics filed an FDA application to gain approval of its cell therapy, deramiocel, designed to treat the heart muscle disease that affects patients with Duchenne muscular dystrophy (DMD), with the FDA providing the therapy priority review to speed its review.
(Source: https://www.capricor.com)
- In November 2024, Regenxbio has a gene therapy, RGX-202, in development in Duchenne muscular dystrophy (DMD) that is progressing to pivotal development, and a BLA submission is planned in 2026. The FDA approval route has aligned with the company on a fast track, with associations considering RGX-202 to be a single-dose treatment that, in some way, could rival the approved Sarepta's emergency, Elevidys, in the DMD segment.
(Source: https://regenxbio.gcs-web.com)
- In November 2024, Cumberland Pharmaceuticals Inc. stated that the U.S. Food and Drug Administration (FDA) has designated Orphan Drug and Rare Pediatric Disease Designations to Ifetroban as a treatment for cardiomyopathy in Duchenne muscular dystrophy (DMD). The company is already carrying out the FIGHT DMDTM Phase II (multi-center, double-blind, placebo-controlled) trial to assess the pharmacokinetics, safety, and efficacy of Ifetroban once a day on the oral route in DMD patients.(Source: https://investor.cumberlandpharma.com)
Market segment
By Treatment
- Molecular-based therapies
- Mutation suppression
- Exon suppression
- Steroid-based therapies
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Others
By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
By Region
- North America
- Asia-Pacific
- Europe
- Latin America
- Middle East & Africa
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content




 sales@precedenceresearch.com
sales@precedenceresearch.com
 +1 804-441-9344
+1 804-441-9344
 Schedule a Meeting
Schedule a Meeting