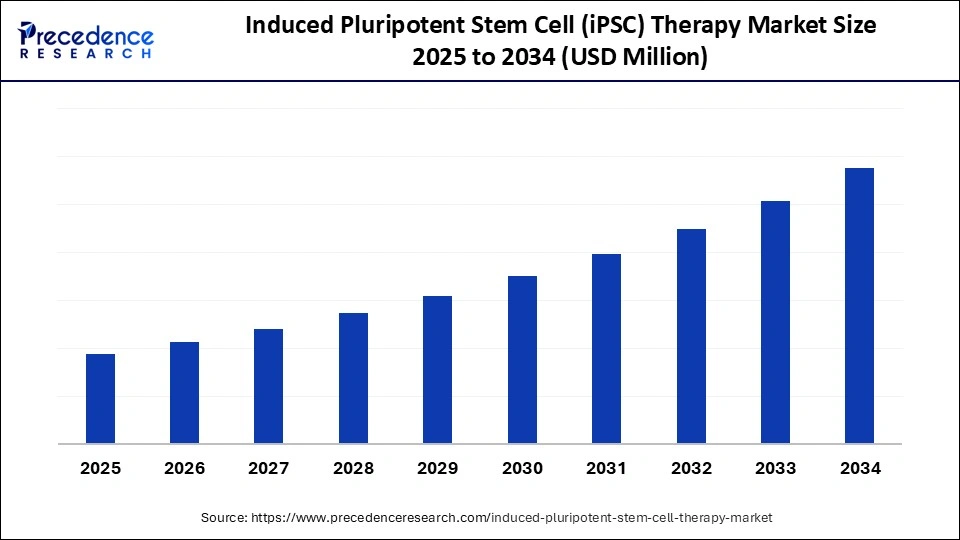
What is Induced Pluripotent Stem Cell (iPSC) Therapy Market Size?
The global induced pluripotent stem cell (iPSC) therapy market growth is fueled by stem-cell R&D funding, clinical translation of iPSC therapeutics and expanding cell-therapy pipelines. The market is witnessing substantial growth due to increasing demand for personalized medicine and regenerative therapies for chronic diseases. Its expansion is further fueled by technological advances that enhance iPSC production efficiency and scalability. Additionally, the integration of AI and machine learning is accelerating research and development, propelling market growth.
Market Highlights
- North America held the largest market share of around 40% in 2024.
- Asia Pacific is expected to grow at the fastest rate from 2025 to 2034.
- By cell type/lineage, the hematopoietic cells segment held the largest market share of about 40% in 2024.
- By cell type/lineage, the hepatocytes segment is expected to witness the fastest growth from 2025 and 2034.
- By application, the regenerative medicine segment dominated the market with around 35% share in 2024.
- By application, the disease modeling segment is expected to grow at the fastest CAGR from 2025 to 2034.
- By product & services, the iPSC-derived cells segment led the market with around 45% share in 2024.
- By product & services, the stem cell banking services segment is expected to register the fastest CAGR during the foreseeable period.
- By end-user, the pharmaceutical & biotech companies segment held the largest market share of about 50% in 2024.
- By end-user, the CRO & CDMOs segment is expected to grow at the fastest CAGR during the foreseeable period.
- By workflow, the reprogramming segment led the market with around 30% share in 2024.
- By workflow, the cell characterization segment is expected to witness the fastest growth during the foreseeable period.
What is Induced Pluripotent Stem Cell (iPSC) Therapy?
The prevalence of chronic diseases is increasing alongside aging populations, driving greater investments in research and development for regenerative medicine. Advancements in patient-specific therapies, which aim to minimize immune rejection, further propel this trend. The induced pluripotent stem cell (iPSC) therapy market is focused on the development and application of iPSCs for therapeutic purposes. iPSCs are reprogrammed somatic cells capable of differentiating into multiple cell types, making them valuable for regenerative medicine, disease modeling, and personalized therapies.
Induced Pluripotent Stem Cell (iPSC) Therapy Market Outlook
- Market Growth Overview: The market is poised for substantial growth between 2025 and 2034, driven by its potential in regenerative medicine, personalized treatments, and drug discovery. Key growth segments include iPSC-derived cells for cardiology, neurology, and oncology, addressing a rising burden of chronic and degenerative diseases.
- Global Expansion: Leading players are expanding their reach globally, targeting regions with favorable regulatory frameworks and high demand, particularly Asia-Pacific (especially Japan) and North America. Strategic partnerships between developers and regional biomanufacturers accelerate clinical translation and market access.
- Major Investors:Significant investment is flowing from venture capital, private equity, and biopharma giants into the iPSC therapy space. Investors are drawn by strong clinical pipelines and the transformative potential of iPSC-based treatments for chronic and genetic disorders, supporting innovations in personalized medicine and cell banking.
- Startup Ecosystem: A maturing startup ecosystem is focused on high-growth niches like developing allogeneic (off-the-shelf) therapies, refining gene-editing techniques like CRISPR for immune evasion, and creating specialized iPSC-derived cell types. Emerging firms are attracting significant funding by offering solutions for large-scale, automated iPSC manufacturing and clinical-grade cell products.
Key Technological Shift in the Induced Pluripotent Stem Cell (iPSC) Therapy Market by AI
Artificial intelligence (AI) is transforming the induced pluripotent stem cell (iPSC) therapy market by speeding up disease modeling and drug discovery through advanced data analysis and high-throughput screening. Also, AI is increasingly used to optimize culture conditions for large-scale iPSC production, analyze genetic and omics data to uncover biological patterns for personalized medicine, and even help predict patient outcomes. These innovations are pushing iPSC therapy toward a more automated, efficient, and precise approach for regenerative medicine, enabling a better understanding of diseases and the creation of personalized cellular environments for drug testing.
Market Scope
| Report Coverage | Details |
| Dominating Region | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Cell Type/Lineage,Application,Product & Services,Workflow, End User and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Application of iPSCs in Drug Discovery and Toxicity Testing
The primary driver of the induced pluripotent stem cell (iPSC) therapy market is the rising application of iPSCs in drug discovery and toxicity testing. Their ability to provide patient-specific human cell models accelerates and improves pharmaceutical development. iPSCs offer physiologically relevant cell models that are genetically matched to individual patients, allowing for personalized drug testing and reducing the reliance on animal models. They are used to model complex diseases such as neurological, cardiovascular, and metabolic disorders, facilitating the discovery of targeted therapies.
Restraint
Inherent Risk of Tumorigenicity
A significant restraint in the induced pluripotent stem cell (iPSC) therapy market is the inherent risk of tumorigenicity, which limits its advancement and clinical adoption. This risk arises from several factors, including the reprogramming process, the use of specific viral vectors, and the potential presence of residual undifferentiated iPSCs in the final product, which can proliferate uncontrollably and form tumors. Some of the initial viral vectors used to deliver reprogramming factors, like the oncogene c-Myc, are known to cause mutations that can lead to cancer.
Opportunity
Development of Off-The-Shelf or Allogeneic Cell Therapies
A key opportunity for the future growth of this market lies in the development of off-the-shelf or allogeneic cell therapies. Instead of relying on patient-specific cells, allogeneic therapies utilize cells from healthy donors that are modified to reduce the risk of immune rejection. This approach allows for standardized, large-scale manufacturing and widespread availability of treatments, helping avoid immune rejection. While allogeneic therapies are expensive, time-consuming, and challenging to scale, their shift towards mass production offers advantages such as improved accessibility and reduced manufacturing time.
Segment Outlook
Cell Type/Lineage Insights
What Made Hematopoietic Cells the Leading Segment in the Market in 2024?
The hematopoietic cells segment led the induced pluripotent stem cell (iPSC) therapy market with around 40% market share in 2024. This is mainly because of the well-established clinical history of hematopoietic stem cell transplantation and the unique benefits of iPSC technology in overcoming key limitations. The short lifespan of mature blood cells means that a fresh, consistent supply is often needed for treatment. iPSCs provide an endless, renewable source of cells for therapies like transfusions or immunotherapy. Hematopoietic cells derived from iPSCs offer a scalable and accessible source of patient-specific cells, reducing issues with donor availability and immune rejection.
The hepatocytes segment is expected to grow at the fastest CAGR during the projection period due to the high demand for liver disease treatments and the limitations of current options. Primary human hepatocytes, traditionally used for research and transplantation, are hard to source and have several limitations. iPSC-derived hepatocytes offer a potentially unlimited supply for liver regeneration, disease modeling, and drug screening, addressing major obstacles that have historically made large-scale production difficult.
Application Insights
How Does the Regenerative Medicine Segment Dominate the Induced Pluripotent Stem Cell (iPSC) Therapy Market?
The regenerative medicine segment dominated the market by holding about 35% share in 2024. This is because iPSCs are perfect for cell replacement therapies since they can be reprogrammed from a patient's own cells, reducing the risk of immune rejection after transplantation. This also removes ethical concerns related to embryonic stem cells and helps address the organ donor shortage. iPSCs can also turn into almost any cell type, making them highly versatile for repairing or replacing damaged or diseased tissues, making them a valuable resource for tissue regeneration in various diseases.
The disease modeling segment is likely to grow at the fastest rate in the upcoming period. This is because it provides patient-specific, human-relevant disease models that overcome the limitations of traditional research methods. This approach allows for a deeper understanding of disease mechanisms and offers a superior platform for drug discovery and screening. It enables researchers to investigate the precise genetic and molecular basis of a patient's condition. This overcomes the limitations of donor cells, which have limited availability and lifespan.
Product & Services Insights
Why Did the iPSC-Derived Cells Segment Lead the Induced Pluripotent Stem Cell (iPSC) Therapy Market?
The iPSC-derived cells segment led the market, holding about 45% share in 2024. This is mainly because iPSCs are not therapeutic in their undifferentiated state; their value lies in being transformed into specific cell types needed for a variety of applications, offering a stable, ethically sound alternative to embryonic stem cells. These applications include regenerative medicine, disease modeling, and drug development, each driving demand for different derived cell types like cardiomyocytes, neurons, and immune cells, to regenerate damaged tissues and organs for conditions ranging from diabetes to liver disease.
The stem cell banking services segment is expected to grow at the fastest CAGR during the forecast period. This is mainly due to the shift from personalized (autologous) to universal (allogeneic) therapies. This change addresses the high costs, complex logistics, and quality control issues tied to producing unique iPSC lines for each patient. Cell banks provide a more affordable, consistent, and scalable solution for therapeutic development by implementing current Good Manufacturing Practice-compliant protocols to produce, store, and distribute high-quality, genetically stable iPSC lines.
End-User Insights
What Made Pharmaceutical & Biotech Companies the Major End-Users?
The pharmaceutical & biotech companies segment held around 50% share of the market in 2024. This is due to their considerable resources, advanced infrastructure, and expertise in navigating the complex and costly process of drug development. Their high levels of R&D and strategic collaborations are crucial for turning iPSC research into commercial products. These companies are well-positioned to commercialize advanced iPSC applications, such as using gene-editing technologies like CRISPR/Cas9 to correct genetic defects in patient-derived cells, speeding up their drug discovery processes.
The CRO & CDMOs segment is expected to grow at the fastest rate during the forecast period. This is because these specialized firms are better equipped to handle the significant technical, logistical, and financial challenges of iPSC manufacturing and clinical translation. Pharmaceutical and biotech companies outsource these complex processes to leverage CROs and CDMOs' expertise, infrastructure, and ability to navigate regulatory hurdles to bring new iPSC therapies to market while reducing risks associated with highly specialized and evolving technology.
Workflow Insights
Why Did the Reprogramming Segment Dominate the Induced Pluripotent Stem Cell (iPSC) Therapy Market?
The reprogramming segment dominated the market by holding about 30% share in 2024. This is mainly because it is the essential starting point for all iPSC-based applications, including disease modeling, drug screening, and cell therapies. Advances in reprogramming techniques, especially safer and more efficient non-integrating methods like messenger RNA (mRNA) reprogramming, have sped up its market growth. The entire iPSC workflow relies heavily on the quality and efficiency of this initial step, with ongoing focus on improving efficiency, reproducibility, and scalability.
The cell characterization segment is expected to expand at the highest CAGR in the near future. This is because it is a critical step to ensure the safety and effectiveness of therapeutic products. Extensive characterization at each stage, from reprogramming to differentiation, is necessary to produce a reproducible and consistent final product. This includes testing for cell viability, pluripotency, and the ability to differentiate into specific cell types, ensuring that the cellular models accurately reflect human physiology.
Regional Insights
How Did North America Lead the Induced Pluripotent Stem Cell (iPSC) Therapy Market in 2024?
North America led the market by capturing around 40% share in 2024. This is due to its strong research infrastructure, significant public and private funding, and a robust network of biotech and pharmaceutical companies. A favorable regulatory environment that encourages clinical trials and innovation also plays a vital role. Large investments in research and commercialization continue to drive growth in the region's efforts. Government agencies like the U.S. National Institutes of Health have invested millions in regenerative medicine, including iPSC projects facilitate clinical translation and provide enhanced support for clinical and preclinical applications.
U.S. Induced Pluripotent Stem Cell (iPSC) Therapy Market Trends
The U.S. plays a major role in the market, primarily due to extensive investment, robust research, and a strong biotech industry. The country dominates the North American market, driving innovation in personalized medicine, regenerative therapies, and drug discovery. A supportive regulatory environment managed by the FDA, along with significant public and private funding, positions the U.S. as a powerhouse for iPSC development, advancing both non-therapeutic and clinical applications globally.
Canada Induced Pluripotent Stem Cell (iPSC) Therapy Market Trends
Canada is evolving as a key player in the market, driven by significant government funding, a collaborative research ecosystem, and strategic initiatives in regenerative medicine. The country's Stem Cell Network acts as a national leader, translating research into clinical applications and supporting the growth of biotech companies. With a robust regulatory framework overseen by Health Canada, advancing iPSC-derived therapies for a wide range of diseases.
Why is Asia Pacific Considered the Fastest-Growing Region in the Induced Pluripotent Stem Cell (iPSC) Therapy Market?
Asia Pacific is expected to experience the fastest growth in the market during the forecast period. This is primarily driven by strong government support, favorable regulations, robust research investments, and rising demand from a large patient population. Countries like Japan, China, and India are leading the charge by funding research, supporting biotech startups, and accelerating clinical trials. With a large and aging population, the prevalence of chronic diseases increases the demand for advanced, regenerative therapeutics. Progress in cell reprogramming, gene editing technologies, and large-scale manufacturing processes is making iPSC therapies more efficient to produce.
India Induced Pluripotent Stem Cell (iPSC) Therapy Market Trends
The market in India is experiencing rapid growth, driven by the country's strong scientific talent and well-established research infrastructure that enable the development of affordable and accessible healthcare solutions. Increased R&D investment, emphasis on personalized medicine, and supportive government regulations in stem cell research, along with collaborations focusing on indigenous iPSC lines, disease modeling, and drug discovery, position India as a significant player in cost-effective regenerative medicine.
What Potentiates the Growth of the Latin America Induced Pluripotent Stem Cell (iPSC) Therapy Market?
The market in Latin America is growing, fueled by a strong focus on early-stage research and clinical applications, alongside rising chronic disease prevalence and increased investment in regenerative medicine by academic institutions and private biotech firms. While challenges such as funding constraints and unclear regulatory frameworks persist, the region holds significant growth potential driven by interest in iPSC technology, drug discovery, and collaborations with international partners.
Brazil Induced Pluripotent Stem Cell (iPSC) Therapy Market Trends
Brazil is leading the iPSC research landscape in Latin America, driven by substantial academic and government-funded initiatives focusing on neurodegenerative diseases, cardiovascular conditions, and personalized medicine. Although clinical therapies are in the early stages, the country is witnessing increased collaborations, private investment, and efforts to establish clear regulatory pathways through agencies such as ANVISA that support the translation of lab-based research into clinical applications.
What Opportunities Exist in the Middle East and Africa for the Induced Pluripotent Stem Cell (iPSC) Therapy Market?
The Middle East and Africa (MEA) presents significant opportunities for market expansion, owing to increasing investments in healthcare infrastructure and biotechnology research. Due to the increasing prevalence of genetic and chronic disorders, countries in the region are building research centers and forming international partnerships. Challenges in the MEA include a lack of specialized infrastructure and a limited skilled workforce; however, the market potential remains strong as governments focus on advanced healthcare solutions and regenerative medicine.
Saudi Arabia Induced Pluripotent Stem Cell (iPSC) Therapy Market Trends
Saudi Arabia plays a vital role in the MEA market, utilizing its National Vision 2030 to make significant investments in biomedical research and advanced therapies. The country emphasizes using iPSCs for regenerative medicine and studying hereditary diseases common in the region. Strategic partnerships with global scientific organizations and substantial government funding aim to develop cutting-edge research facilities, positioning Saudi Arabia as a center for stem cell research and upcoming clinical trials.
What Makes Europe a Notably Growing Area in the Induced Pluripotent Stem Cell (iPSC) Therapy Market?
Europe is experiencing notable growth in the market, supported by advanced research infrastructure, a strong academic foundation, and substantial government funding programs like Horizon Europe. The market is driven by an emphasis on personalized medicine, drug screening, and the development of cell therapies for various diseases. Strict yet clear regulatory frameworks established by the European Medicines Agency (EMA) facilitate the development and commercialization of new therapies, with many academic spin-offs and biotech companies actively involved in clinical trials.
Germany Induced Pluripotent Stem Cell (iPSC) Therapy Market Trends
Germany dominates the European market, emphasizing thorough research, the development of strong manufacturing protocols, and the use of iPSCs for toxicological testing and disease modeling. Home to top research institutes and a robust biotech sector, the country benefits from substantial public and private funding. The German market is dedicated to turning basic research into clinical treatments for conditions like Parkinson's disease and heart failure, and it leads in creating automated, scalable iPSC production technologies.
Country-level Investments & Funding Trends for Induced Pluripotent Stem Cell (iPSC) Therapy Market
|
Country |
Noteworthy Funding/Investment & Status |
|
U.S. |
Significant government funding (NIH >$2B recently, $79M iPSC-specific in 2021); Robust VC activity |
|
Japan |
Government pledge: 110 billion yen ($1.13 billion); Private funding: Heartseed Inc. raised $74 million (2023) |
|
China |
Cell therapy funding: $2.5 billion (2023); R&D Investment: >$3 billion (2021-2023) |
|
South Korea |
Cell Therapy funding: >$99.2 million (past 10 years); Saw 34.02% drop in funding (2022 vs 2021) |
|
Europe |
Market value: $546.24 million (2024); Collaborative EU funding and public-private partnerships (e.g., EBiSC) |
Induced Pluripotent Stem Cell (iPSC) Therapy Regulatory Landscape: Global Regulations
|
Country |
Regulatory Body |
Key Regulations/Frameworks |
Initiatives/Approach |
|
Japan |
Pharmaceuticals and Medical Devices Agency (PMDA) |
Act on the Safety of Regenerative Medicine; fast-track conditional approval |
Strong government funding, leading clinical trials, and dedicated research centers like CiRA. |
|
U.S. |
Food and Drug Administration (FDA), CBER |
Biologics License Application (BLA) pathway; Investigational New Drug (IND) applications |
Significant industry investment, NIH funding, and a flexible regulatory environment. |
|
EU |
European Medicines Agency (EMA) |
Advanced Therapy Medicinal Products (ATMP) framework; rigorous standards |
Emphasis on standardized testing methods; rigorous, harmonized approval process across member states. |
|
China |
National Stem Cell Research Supervision and Coordination Committee |
Evolving guidelines and standards for stem cell research |
Strong government investment and provincial subsidies for manufacturing facilities. |
|
South Korea |
Ministry of Food and Drug Safety |
Advanced Regenerative Medicine Act (2019) |
Accelerates review of new therapies; leverages advanced manufacturing technology for bioreactors. |
Value Chain Analysis
Cell Reprogramming & iPSC Generation
Somatic cells (typically skin or blood cells) are reprogrammed into pluripotent stem cells using non-integrative or integrative methods such as Sendai virus, episomal vectors, or mRNA-based techniques. This stage is highly technical, requiring stringent quality control to ensure genomic stability, pluripotency, and reproducibility of iPSC lines suitable for therapeutic use.
- Key Players: REPROCELL, FUJIFILM Cellular Dynamics, Inc. (FCDI), Creative Bioarray, Cyagen.
iPSC Expansion & Differentiation
Once generated, iPSCs are expanded in vitro under defined conditions and differentiated into specific cell types such as cardiomyocytes, neurons, or pancreatic beta cells, depending on therapeutic needs. The expansion and lineage-specific differentiation processes must be optimized for scalability, purity, and functionality, which are essential for clinical translation and regulatory approval.
- Key Players: Ncardia, Axol Bioscience, BrainXell, Creative Biolabs.
Preclinical Research & Disease Modeling
iPSC-derived cells are used in preclinical studies for drug screening, toxicity testing, and disease modeling, providing a human-relevant alternative to animal models. This enables pharmaceutical companies to better predict efficacy and safety profiles, reducing attrition rates and accelerating drug development pipelines.
- Key Players: Evotec SE, Fate Therapeutics, Curi Bio and NEXEL (Celogics), and iXcells Biotechnologies.
Clinical Trials & Regulatory Compliance
Therapeutic iPSC products undergo phased clinical trials to validate safety, dosage, and efficacy for targeted indications such as Parkinson's disease, macular degeneration, or heart failure. Regulatory bodies such as the FDA, EMA, and PMDA enforce strict guidelines for cell sourcing, manufacturing, and patient administration, making compliance a critical part of this stage.
- Key Players: Cynata Therapeutics, BlueRock Therapeutics, Fate Therapeutics, Astellas Pharma Inc.
Manufacturing & GMP-Scale Production
iPSC therapies require Good Manufacturing Practice (GMP)-compliant facilities capable of large-scale, sterile, and reproducible production of cell-based products. This stage involves bioprocess optimization, cryopreservation, and packaging, ensuring product consistency across batches and enabling broader commercialization.
- Key Players: FUJIFILM Cellular Dynamics, Inc. (FCDI), Treefrog Therapeutics, REPROCELL, Catalent.
Top Companies Competing in the Induced Pluripotent Stem Cell (iPSC) Therapy Market
Tier I – Major Players (~40–50% of Total Market Share)
These are the dominant global companies with significant individual shares, collectively accounting for roughly 40–50% of the overall iPSC therapy market revenue. They lead in technology innovation, scale, and commercialization.
- Fujifilm Cellular Dynamics, Inc.
A pioneer in iPSC technology, Fujifilm Cellular Dynamics produces high-quality human iPSC-derived cells used in drug discovery, disease modeling, and regenerative medicine applications.
- Astellas Pharma Inc.
Astellas leverages its strong pharmaceutical expertise to develop iPSC-based regenerative therapies, focusing on novel treatments for conditions like blindness and neurodegenerative diseases.
- Thermo Fisher Scientific Inc.
Thermo Fisher provides comprehensive iPSC research tools and reagents, enabling scientists and biopharma companies to accelerate iPSC generation, characterization, and therapeutic development.
- Takara Bio Inc.
Takara Bio specializes in innovative cell reprogramming technologies and offers advanced iPSC culture and differentiation products to support regenerative medicine and drug discovery efforts.
Tier II – Mid-Level Contributors (30–35% of Market Share)
These companies have a solid presence and growing influence in the market. They provide specialized iPSC products, research tools, or regional solutions and together contribute about 30–35% of the market.
- Lonza Group AG
Lonza provides advanced cell therapy manufacturing services and scalable iPSC production platforms, enabling the commercialization of iPSC-based regenerative therapies.
- Merck KGaA
Merck offers a wide portfolio of reagents, media, and tools for iPSC culture and differentiation, supporting research and clinical development in regenerative medicine.
- Bio-Techne Corporation
Bio-Techne supplies specialized proteins, antibodies, and assay kits that facilitate iPSC research, quality control, and therapeutic development.
- Stemcell Technologies
Stemcell Technologies delivers innovative cell culture media and tools designed to enhance the growth, maintenance, and differentiation of iPSCs for research and clinical applications.
- Cellular Dynamics International
A subsidiary of Fujifilm, Cellular Dynamics International focuses on producing standardized human iPSC-derived cells for drug screening, disease modeling, and cell therapy development.
Tier III – Niche and Regional Players (~15–20% of Market Share
Smaller or regionally focused players, with niche offerings or emerging capabilities. Individually modest, collectively they hold 15–20% of the market.
- REPROCELL USA Inc.
- Ncardia
- ViaCyte, Inc.
- Kyoto University (research-focused)
- Pluricell Biotech
- Other emerging startups and local manufacturers
Exclusive Analysis on the Induced Pluripotent Stem Cell (iPSC) Therapy Market
The global induced pluripotent stem cell (iPSC) therapy market is transitioning from a primarily research-centric phase to a commercialization-oriented trajectory, driven by the convergence of advanced reprogramming technologies, scalable manufacturing platforms, and a pressing demand for patient-specific, regenerative therapies. With the limitations of allogeneic and embryonic stem cell-based approaches increasingly apparent, particularly in terms of ethical constraints and immune compatibility, iPSCs represent a paradigm shift in personalized medicine and disease modeling.
The market is witnessing accelerated momentum fueled by rising chronic disease prevalence, an aging demographic, and intensifying investment from both public and private sectors in cell-based therapeutics. Regulatory receptiveness, particularly in the U.S., Japan, and parts of the EU, toward expedited clinical pathways for cell and gene therapies further enhances the market's investability.
Opportunities lie most prominently in autologous cell therapy development, where iPSCs can be derived from the patient's own somatic cells, thereby reducing immunogenic risk while enabling highly targeted therapeutic intervention. Moreover, the application of iPSCs in drug discovery and toxicology screening is poised to disrupt preclinical R&D models by improving translational accuracy and lowering attrition rates.
Strategic alliances between biopharmaceutical giants and specialized iPSC platform companies are intensifying, aimed at overcoming scalability, reproducibility, and GMP-compliance challenges. Additionally, the incorporation of CRISPR-based gene editing with iPSC technology is unlocking new frontiers in the treatment of rare genetic disorders, creating a high-impact segment within the broader cell therapy space.
In sum, the induced pluripotent stem cell (iPSC) therapy market is positioned at the inflection point of scientific innovation and commercial scalability. Stakeholders capable of navigating technical complexities, regulatory nuances, and capital-intensive development cycles stand to capture significant value in a market that is not only growing but redefining the future of regenerative medicine.
Recent Developments
- In June 2025, XellSmart Biomedical Co., Ltd. (Suzhou/Shanghai, China), a leading biotechnology firm focused on iPSC-derived cell therapies, received seven Investigational New Drug approvals from the NMPA of China and the U.S. FDA. Their trials target CNS diseases with significant unmet needs, including Parkinson's Disease, Spinal Cord Injury, and Amyotrophic Lateral Sclerosis.
- In April 2025, Fate Therapeutics, Inc. announced that the FDA granted RMAT designation to FT819, an iPSC-derived CAR T-cell therapy for moderate to severe systemic lupus erythematosus. This designation highlights the therapy's potential to meet the needs of a diverse lupus patient population by providing accessible and cost-effective treatment. (Source: https://ir.fatetherapeutics.com)
- In May 2025, REPROCELL introduced StemEdit™ Human iPSC cell lines, designed specifically for research purposes, which lack HLA class 1 or both classes 1 and 2 expressions. This innovation aids researchers in studying immune interactions with iPSCs.
Segments Covered in the Report
By Cell Type / Lineage
- Hematopoietic Cells
- Hematopoietic Stem Cells (HSCs)
- Erythrocytes
- Leukocytes
- Platelets
- Hepatocytes
- Liver Parenchymal Cells
- Non-Parenchymal Cells
- Cardiomyocytes
- Atrial Cardiomyocytes
- Ventricular Cardiomyocytes
- Neurons
- Dopaminergic Neurons
- Cholinergic Neurons
- Glutamatergic Neurons
- Endothelial Cells
- Vascular Endothelial Cells
- Lymphatic Endothelial Cells
- Others
- Astrocytes
- Fibroblasts
- Pancreatic Beta Cells
By Application
- Regenerative Medicine
- Tissue Repair
- Organ Regeneration
- Drug Development & Discovery
- High-Throughput Screening
- Lead Compound Identification
- Disease Modeling
- Genetic Disease Models
- Acquired Disease Models
- Toxicity Screening
- Cardiotoxicity
- Hepatotoxicity
- Cell Therapy
- Autologous Therapies
- Allogeneic Therapies
By Product & Services
- iPSC-derived Cells
- Cardiomyocytes
- Neurons
- Hepatocytes
- Reprogramming Kits
- Non-integrating Kits
- Integrating Kits
- Stem Cell Banking Services
- Cryopreservation
- Cell Line Maintenance
- Culture Media & Supplements
- Basal Media
- Growth Factors
- Instruments & Consumables
- Bioreactors
- Microplates
By End-User
- Pharmaceutical & Biotech Companies
- CROs & CDMOs
- Academic & Research Institutes
- Hospitals & Clinics
By Workflow
- Reprogramming
- Cell Culture
- Cell Characterization / Analysis
- Engineering
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
 Get a Sample
Get a Sample
 Table Of Content
Table Of Content